Evaluation of the Treatment Process of Landfill Leachate Using the Toxicity Assessment Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Physicochemical Tests
2.3. Experimental Device
2.4. Toxicity Tests
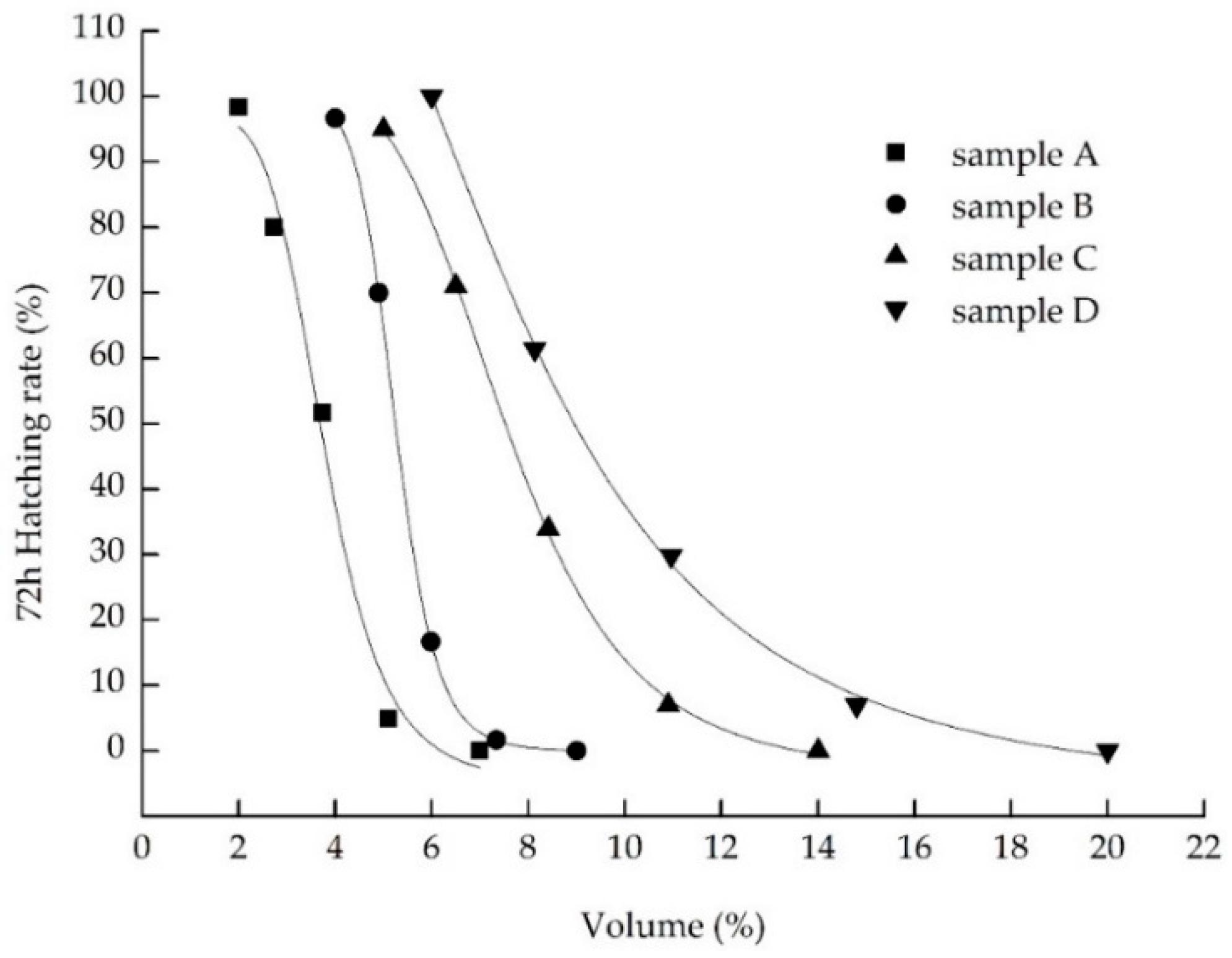
2.4.1. Zebrafish Toxicity Test
2.4.2. Luminous Bacteria Toxicity Test
2.4.3. Methods of Toxicity Evaluation
3. Results
3.1. Coagulation and Sedimentation Process
3.2. Anaerobic Process (Sludge Acclimation and Up-Flow Anaerobic Sludge Bed (UASB) Start)
3.3. Electrochemical Oxidation Process
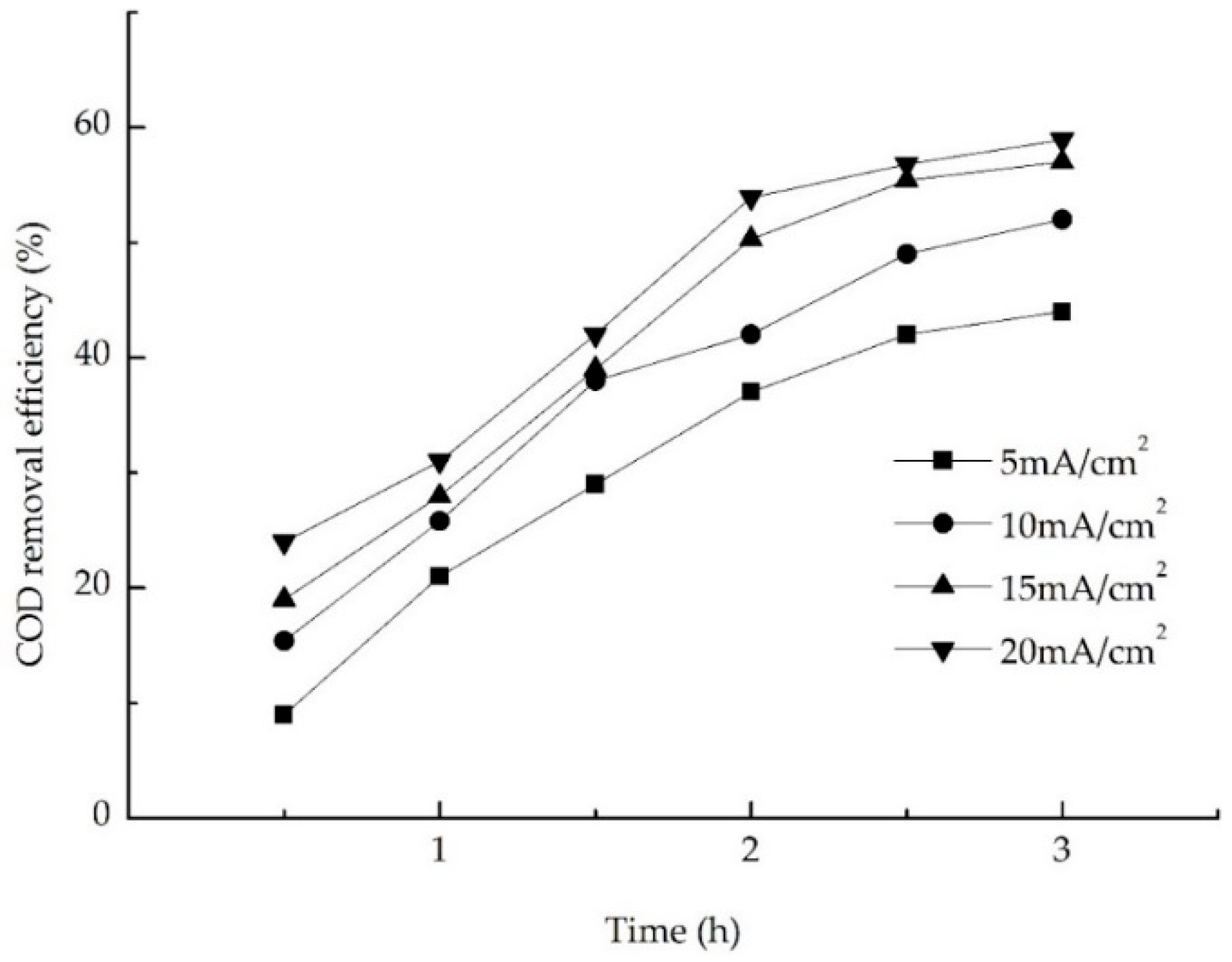
3.3.1. Effect of Current Density on Electrochemical Oxidation
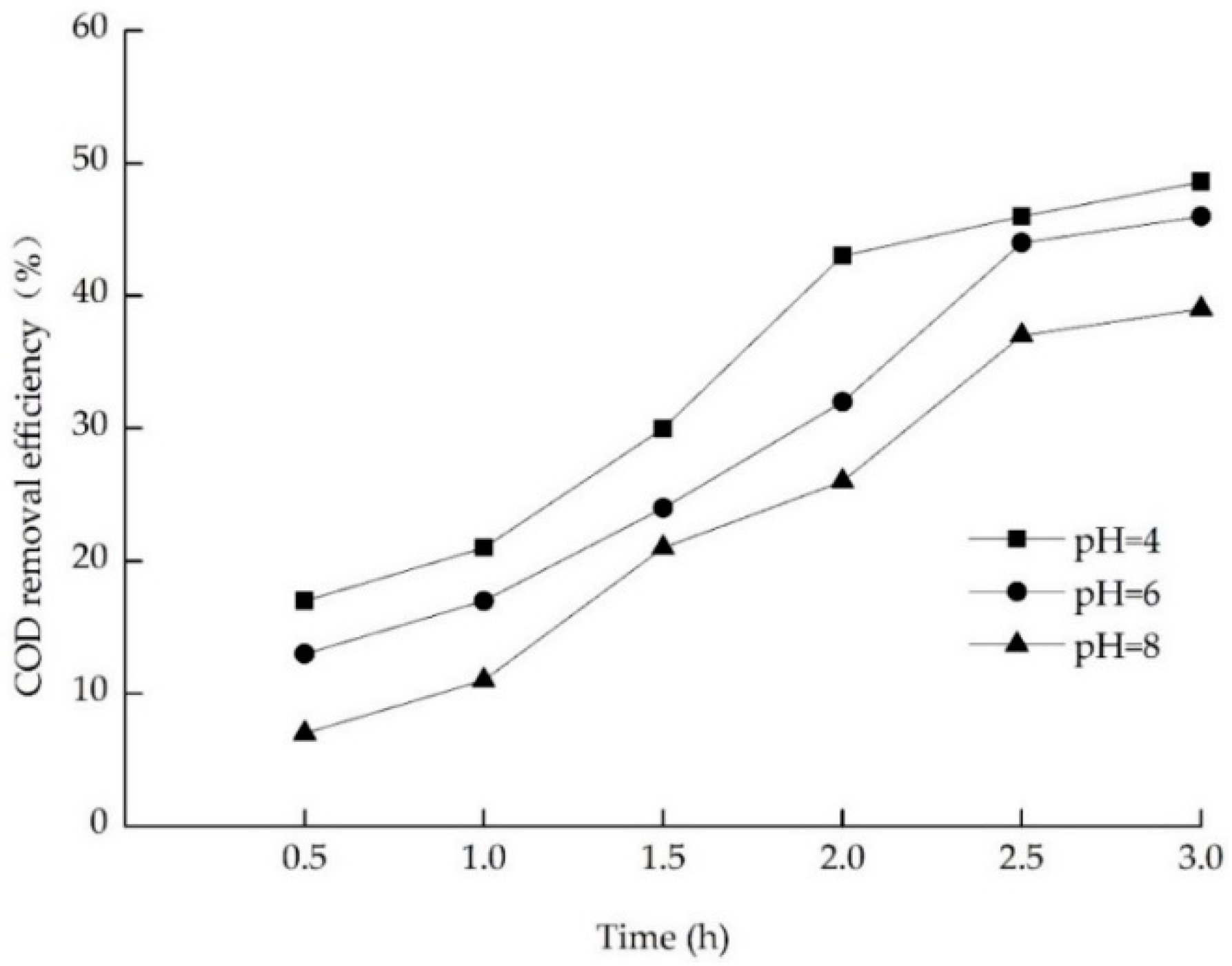
3.3.2. Effect of pH on Electrochemical Oxidation
3.4. Aerobic Process
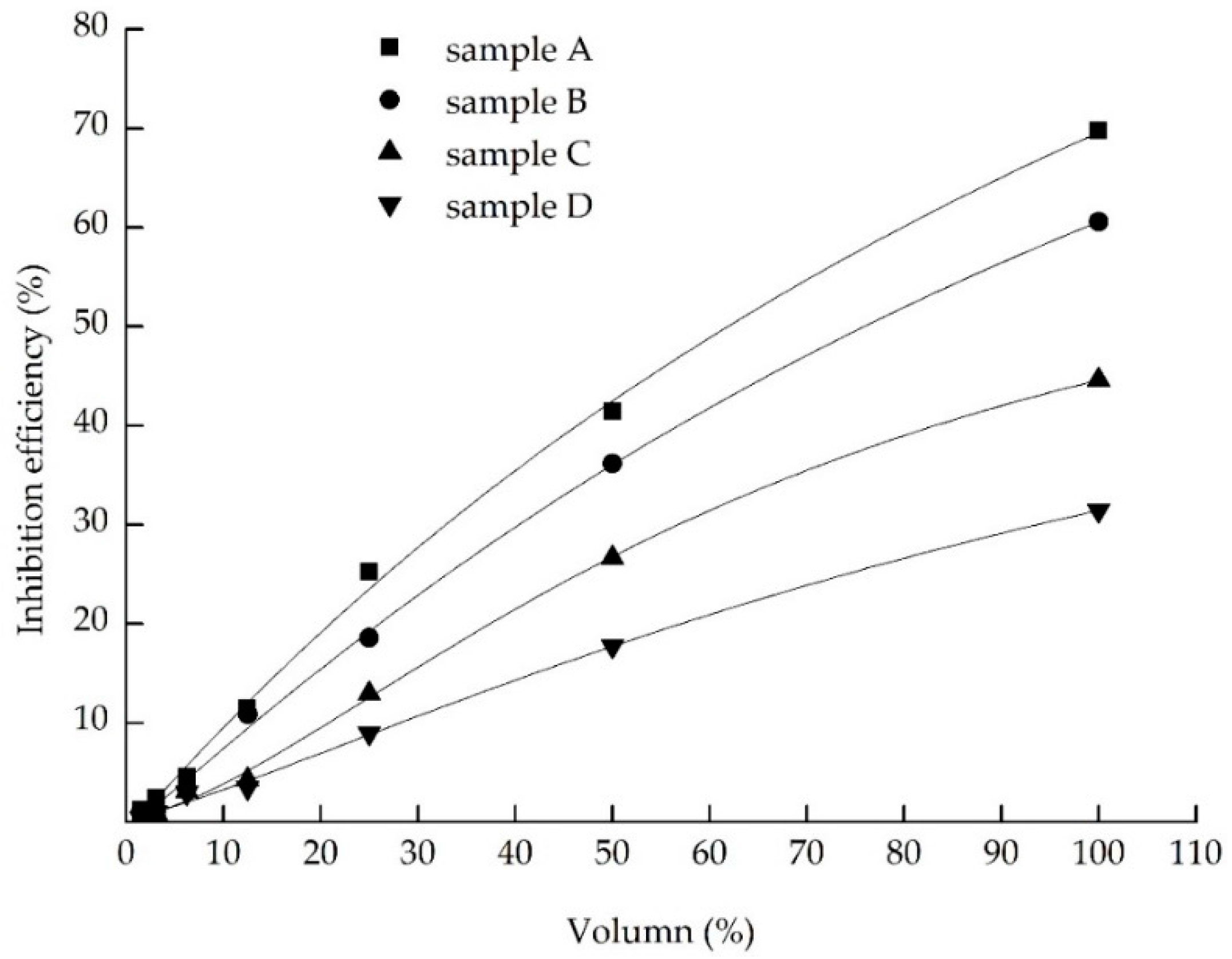
3.5. Combined Landfill Leachate Treatment
4. Discussion
4.1. Reduction of Physicochemical Characters and Toxicity in the Landfill Leachate
4.2. Analyze the Effect of Different Treatment Process
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Song, J.; Guo, J.; Wang, Z.; Feng, Q. Landfill leachate treatment using electrocoagulation. Procedia Environ. Sci. 2011, 10, 1159–1164. [Google Scholar] [CrossRef]
- Kalčíková, G.; Zupančič, M.; Levei, E.A.; Miclean, M.; Englande, A.J. Application of multiple toxicity tests in monitoring of landfill leachate treatment efficiency. Environ. Monit. Assess. 2015, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Moody, C.M.; Townsend, T.G. Ozonation pretreatment for stabilized landfill leachate high-pressure membrane treatment. Desalination 2014, 344, 163–170. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Silva, S.M.C.P.; Martinez, C.B.R. Assessment of domestic landfill leachate toxicity to the Asian clam Corbicula fluminea via biomarkers. Ecotoxicol. Environ. Saf. 2014, 103, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Fauziah, S.H.; Lzzati, M.N.; Agamuthu, P. Toxicity on Anabas testudineus: A case study of sanitary landfill leachate. Procedia Environ. Sci. 2013, 18, 14–19. [Google Scholar] [CrossRef]
- Kalčíková, G.; Vávrová, M.; Zagorc-Končan, J. Evaluation of the hazardous impact of landfill leachates by toxicity and biodegradability tests. Environ. Technol. 2011, 32, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Dailianis, S. Investigation of landfill leachate toxic potency: An integrated approach with the use of stress indices in tissues of mussels. Aquat. Toxicol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Toufexi, E.; Tsarpali, V.; Efthimioub, I.; Vidali, M.S.; Vlastosb, D.; Vlastosb, S. Environmental and human risk assessment of landfill leachate: an integrated approach with the use of cytotoxic and genotoxic stress indices in mussel and human cells. J. Hazard. Mater. 2013, 260, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Kamilari, M.; Dailianis, S. Seasonal alterations of landfill leachate composition and toxic potency in semi-arid regions. J. Hazard. Mater. 2012. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Quan, X.; Chen, B.; Cheng, Z. Electrochemical treatment of biologically treated leachate from municipal solid waste incinerator. Chin. J. Environ. Eng. 2013, 7, 4823–4828. (In Chinese) [Google Scholar]
- Li, T.; Wang, H. Treatment of landfill leachate by electrochemical oxidation and anaerobic process. Water Environ. Res. 2007, 79, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chang, J.E.; Chung, C.T. Electrochemical oxidation combined with physical-chemical pretreatment processes for the treatment of refractory landfill leachate. Environ. Eng. Sci. 2001, 18, 369–379. [Google Scholar] [CrossRef]
- Cossu, R.; Polcaro, A.M.; Mascia, M. Electrochemical treatment of landfill leachate: Oxidation at Ti/PbOâ and Ti/SnOâ anodes. Environ. Sci. Technol. 1998, 32, 3570–3573. [Google Scholar] [CrossRef]
- Linares-Hernández, I.; Barrera-Díaz, C.; Roa-Morales, G.; Bilyeu, B.; Ureña-Núñez, F. Influence of the anodic material on electrocoagulation performance. Chem. Eng. J. 2009, 148, 97–105. [Google Scholar] [CrossRef]
- Lidia, S.; Kaul, S.N.; Rao, N.N.; Satyanarayan, S. Influence of anode material on electrochemical oxidation for the treatment of tannery wastewater. Water Res. 2005, 39, 1601–1613. [Google Scholar]
- Wang, Q.G.; Chen, L.E.; Pei-Fei, F.U.; Jiang, W.L.; Han, Y. Study on the treatment of concentrated water from nanofiltration of bio-treated landfill leachate by caustic soda-soda ash process, coagulation-sedimentation and electrochemical oxidation process. Environ. Sci. Technol. 2014, 9, 1308–1312. [Google Scholar]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl. Catal. B-Environ. 2015, 176, 183–200. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B-Environ. 2009, 87, 105–145. [Google Scholar]
- Zhang, J.; Chen, S.; Zhang, Y.; Quan, X.; Zhao, H. Reduction of acute toxicity and genotoxicity of dye effluent using Fenton-coagulation process. J. Hazard. Mater. 2014, 274, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alba, A.R.; Hernando, D.; Agüera, A.; Cáceres, J.; Malato, S. Toxicity assays: A way for evaluating AOPs efficiency. Water Res. 2002, 36, 4255–4262. [Google Scholar] [CrossRef]
- Meriç, S.; Selçuk, H.; Belgiorno, V. Acute toxicity removal in textile finishing wastewater by Fenton’s oxidation, ozone and coagulation–flocculation processes. Water Res. 2005, 39, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Nie, E.; Xu, J.; Yan, S.; Cooper, W.J. Degradation of Diclofenac by advanced oxidation and reduction processes: Kinetic studies, degradation pathways and toxicity assessments. Water Res. 2013, 47, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.L. Bioassays for the evaluation of landfill leachate toxicity. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Bila, D.M.; Montalvão, A.F.; Silva, A.C.; Dezotti, M. Ozonation of a landfill leachate: evaluation of toxicity removal and biodegradability improvement. J. Hazard. Mater. 2005, 117, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Butler, C.A.; Timperley, M.H.; Evans, C.W. Formation of copper complexes in landfill leachate and their toxicity to zebrafish embryos. Environ. Toxicol. Chem. 2000, 19, 1397–1402. [Google Scholar] [CrossRef]
- Irons, T.D.; Macphail, R.C.; Hunter, D.L.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qian, L.; Ling, C.; Zhang, A.; He, J. Toxicity of surface water from Huangpu River to luminous bacteria (Vibrio qinghaiensis, SP. Q67) and zebrafish (D. rerio) embryos. Ecotoxicol. Environ. Saf. 2015, 112, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-Operation and Development (ISO). Water Quality-Determination of the Acute Letha l Toxicity of Substances to a Freshwater Fish [Brachy D. rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]-Part 1: Static Method; ISO7346-1; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Kong, Z.M.; Yang, L.Y.; Yin, D.Q. Modern Techniques & Methods in Environment Biological Experiments; China Environmental Science Press: Beijing, China, 2005; pp. 2–6. [Google Scholar]
- Organization for Economic Co-Operation and Development (ISO). Water Quality-Determinationof the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria; ISO 11348-3; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Li, Y.F.; Yang, Y.; Wang, J. Treatment of Landfill Leachate by Composite Coagulant of Polyaluminum Chloride and Polyacrylamide. Ind Saf. Environ. Prot. 2011, 37, 9–10. [Google Scholar]
- Dai, J.G.; Song, Q.W.; Dai, J.K. Effect of pH value on electrochemical oxidation of landfill leachate. Environ. Eng. 2012, 30, 55–58. (In Chinese) [Google Scholar]
- Gao, X.; Li, P.; Wu, J. Combination process for advanced treatment of biotreatment effluent of landfill leachate. Chin. J. Environ. Eng. 2014, 8, 2376–2379. (In Chinese) [Google Scholar]
- Rocha, J.H.B.; Gomes, M.S.; Fernandes, N.S.; Silva, D.R.D.; Martínez-Huitle, C.A. Application of electrochemical oxidation as alternative treatment of produced water geneefficiencyd by Brazilian petrochemical industry. Fuel Process. Technol. 2012, 96, 80–87. [Google Scholar] [CrossRef]
- Gotvajn, A.Z.; Tisler, T.; Zagorckoncan, J. Comparison of different treatment strategies for industrial landfill leachate. J. Hazard. Mater. 2009, 162, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; He, P.J.; Shao, L.M.; Lee, D.J. Heavy metals mobility in full-scale bioreactor landfill: Initial stage. Chemosphere 2008, 70, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato-Garcia, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.D.; Bisschops, I.A.E.; Cervantes, F.J.; Lier, J.B.V. Effect of different redox mediators during thermophilic azo dye reduction by anaerobic granular sludge and comparative study between mesophilic (30 °C) and thermophilic (55 °C) treatments for decolourisation of textile wastewaters. Chemosphere 2004, 55, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.D.; Prieto-Rodríguez, L.; Sanctis, M.D.; Iaconi, C.D.; Malato, S. Landfill leachate treatment: Comparison of standalone electrochemical degradation and combined with a novel biofilter. Chem. Eng. J. 2015, 288, 87–98. [Google Scholar] [CrossRef]
- Trapido, M. Combination of ozonation and the fenton processes for landfill leachate treatment: Evaluation of treatment efficiency. Ozone-Sci. Eng. 2009, 31, 28–36. [Google Scholar]
- Wiegand, C.; Pflugmacher, S.; Oberemm, A.; Meems, N.; Beattie, K.A. Uptake and effects of microcystin-LR on detoxication enzymes of early life stages of the zebrafish (D. rerio). Environ. Toxicol. 1999, 14, 89–95. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S.; Oberemm, A.; Steinberg, C. Activity development of selected detoxication enzymes during the ontogenesis of the zebrafish (D. rerio). Int. Rev. Hydrobiol. 2000, 5, 413–422. [Google Scholar] [CrossRef]
- Zhang, N.C.; Deng, Y.M.; Quan, X.; Chen, S. Evaluation of the detoxication efficiencies for acrylonitrile wastewater treated by a combined anaerobic oxic-aerobic biological fluidized tank (A/O-ABFT) process: Acute toxicity and zebrafish embryo toxicity. Chemosphere 2016, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A. Toxicity identification evaluation of leachates from municipal solid waste landfills: A multispecies approach. Chemosphere 2003, 52, 85–94. [Google Scholar] [CrossRef]



| Model Organisms | Raw | Coagulation and Sedimentation | Anaerobic | Electrochemical Oxidation | Aerobic |
|---|---|---|---|---|---|
| Zebrafish embryos | 0.50% | 2.00% | 4.00% | 5.00% | 6.00% |
| 0.89% | 2.73% | 4.90% | 6.50% | 8.13% | |
| 1.58% | 3.74% | 5.98% | 8.41% | 10.96% | |
| 2.82% | 5.11% | 7.34% | 10.90% | 14.80% | |
| 5.00% | 7.00% | 7.00% | 14.00% | 20.00% | |
| Zebrafish larvae | 1.40% | 1.50% | 2.00% | 4.00% | 6.00% |
| 1.49% | 1.78% | 2.52% | 4.76% | 7.14% | |
| 1.59% | 2.12% | 3.16% | 5.66% | 8.49% | |
| 1.69% | 2.52% | 3.98% | 6.73% | 10.09% | |
| 1.80% | 3.00% | 5.00% | 8.00% | 12.00% |
| Parameter (mg/L) | Raw Landfill Leachate |
|---|---|
| CODcr | 1400 ± 72.39 |
| NH4+-N | 394 ± 24.17 |
| TP | 1.51 ± 0.33 |
| TN | 516 ± 20.98 |
| pH | 6.55 ± 0.34 |
| Cu | 0.026 ± 0.00162 |
| Zn | 1.1651 ± 0.00015 |
| Cd | 0.0086 ± 0.0008 |
| Cr | 0.2468 ± 0.00133 |
| Ni | 0.157 ± 0.00089 |
| Source | CODcr | NH4+-N | Cd | Cr | Ni | Zn | Cu |
|---|---|---|---|---|---|---|---|
| Raw | 1400 ± 72.39 | 394 ± 24.17 | 0.0086 ± 0.0008 | 0.2468 ± 0.00133 | 0.157 ± 0.00089 | 1.1651 ± 0.00015 | 0.026 ± 0.00162 |
| Sample A | 607 ± 30.76 | 340 ± 36.88 | 0.0015 ± 0.00033 | 0.077 ± 0.00769 | 0.15 ± 0.00261 | 0.0196 ± 0.00046 | - |
| Sample B | 571 ± 16.85 | 315 ± 30 | - | 0.053 ± 0.00141 | 0.072 ± 0.00522 | 0.002 ± 0.00089 | - |
| Sample C | 220 ± 31.62 | 83 ± 5.10 | - | 0.040 ± 0.00261 | 0.0136 ± 0.0008 | - | - |
| Sample D | 90 ± 3.69 | 10 ± 1.10 | - | 0.031 ± 0.00551 | 0.0102 ± 0.00194 | - | - |
| Toxicity Test | Sample | Parameter | Value (%) | 95% CI | TU |
|---|---|---|---|---|---|
| Zebrafish embryos | Raw | 72 h hatching rate (Vol. %) | 1.12 | 0.85–1.56 | 89.3 |
| A | 3.66 | 0.26–7.29 | 27.3 | ||
| B | 5.25 | 5.22–5.68 | 19.0 | ||
| C | 7.49 | 6.59–8.46 | 13.4 | ||
| D | 8.95 | 7.22–11.14 | 11.2 | ||
| Zebrafish embryos | Raw | 72 h malformation rate (Vol. %) | 1.33 | 0.92–1.85 | 75.2 |
| A | 4.68 | 3.95–5.12 | 21.4 | ||
| B | 5.41 | 5.08–5.75 | 18.5 | ||
| C | - | - | - | ||
| D | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, A.; Cai, Q.; Zhao, Y.; Guo, Y.; Zhao, L. Evaluation of the Treatment Process of Landfill Leachate Using the Toxicity Assessment Method. Int. J. Environ. Res. Public Health 2016, 13, 1262. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13121262
Qiu A, Cai Q, Zhao Y, Guo Y, Zhao L. Evaluation of the Treatment Process of Landfill Leachate Using the Toxicity Assessment Method. International Journal of Environmental Research and Public Health. 2016; 13(12):1262. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13121262
Chicago/Turabian StyleQiu, Aifeng, Qiang Cai, Yuan Zhao, Yingqing Guo, and Liqian Zhao. 2016. "Evaluation of the Treatment Process of Landfill Leachate Using the Toxicity Assessment Method" International Journal of Environmental Research and Public Health 13, no. 12: 1262. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13121262






