Impact of Geographic Location on Vitamin D Status and Bone Mineral Density
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Population

2.2. Laboratory and Nutritional Assessment
2.3. Lifestyle Questionnaires
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Study Subjects Categorized by Region and Age
| Men (n = 7064) | ||||
| North (37° N) | Middle (36° N) | South (33° N~36° N) | p | |
| Numbers, n (%) | 3428 (48.5) | 824 (11.7) | 2811 (39.8) | |
| Age (years) | 39.9 (0.4) | 44.0 (1.0) | 41.3 (0.6) | <0.001 |
| Body weight (kg) | 69.9 (0.3) | 69.0 (0.7) | 68.0 (0.3) | <0.001 |
| BMI (kg/m2) | 24.0 (0.1) | 24.0 (0.2) | 23.5 (0.1) | <0.001 |
| Fat mass (kg) | 16.1 (0.2) | 15.5 (0.3) | 14.8 (0.2) | <0.001 |
| Calcium intake (mg/d) | 570.8 (8.3) | 550.8 (18.9) | 567.9 (9.5) | 0.629 |
| 25OHD (nmol/L) | 46.0 (0.7) | 52.7 (1.7) | 51.1 (0.9) | <0.001 |
| FNBMD (g/cm2) | 0.834 (0.003) | 0.808 (0.007) | 0.827 (0.004) | 0.004 |
| LSBMD (g/cm2) | 0.959 (0.003) | 0.949 (0.007) | 0.963 (0.004) | 0.244 |
| Smoking *, n (% †) | ||||
| Current | 1336 (44.1) | 312 (41.5) | 1156 (45.6) | <0.001 |
| Past and never | 1696 (55.9) | 440 (58.5) | 1380 (54.4) | |
| Alcohol intake *, n (% †) | ||||
| Yes | 2282 (75.7) | 552 (73.5) | 1887 (74.4) | <0.001 |
| No | 733 (24.3) | 199 (26.5) | 648 (25.6) | |
| Physical activity *, n (% †) | ||||
| Yes | 329 (10.9) | 146 (19.4) | 375 (14.8) | <0.001 |
| No | 2701 (89.1) | 606 (80.6) | 2160 (85.2) | |
| Occupation *, n (% †) | ||||
| Indoor workers | 2904 (84.7) | 687 (83.4) | 2423 (86.2) | <0.001 |
| Outdoor workers | 243 (7.1) | 87 (10.6) | 192 (6.8) | |
| No jobs | 282 (8.2) | 50 (6.1) | 196 (7.0) | |
| Women (n = 6779) | ||||
| North (37° N) | Middle (36° N) | South (33° N~36° N) | p | |
| Numbers, n (%) | 3182 (46.9) | 826 (12.2) | 2771 (40.9) | |
| Age (years) | 37.0 (0.4) | 42.6 (1.5) | 42.5 (0.7) | 0.001 |
| Body weight (kg) | 56.5 (0.2) | 56.4 (0.3) | 56.5 (0.2) | 0.975 |
| BMI (kg/m2) | 22.7 (0.1) | 23.0 (0.2) | 22.9 (0.1) | 0.173 |
| Fat mass (kg) | 18.9 (0.2) | 18.8 (0.3) | 18.4 (0.2) | 0.079 |
| Calcium intake (mg/d) | 448.0 (6.7) | 400.3 (10.4) | 448.7 (8.9) | <0.001 |
| 25OHD (nmol/L) | 39.7 (0.6) | 44.1 (1.5) | 44.6 (0.8) | <0.001 |
| FNBMD (g/cm2) | 0.725 (0.003) | 0.706 (0.009) | 0.720 (0.004) | 0.132 |
| LSBMD (g/cm2) | 0.922 (0.004) | 0.892 (0.010) | 0.918 (0.004) | 0.029 |
| Smoking *, n (% †) | ||||
| Current | 196 (6.8) | 43 (5.8) | 108 (4.3) | <0.001 |
| Past and never | 2668 (93.2) | 695 (94.2) | 2420 (95.7) | |
| Alcohol intake *, n (% †) | ||||
| Yes | 1178 (41.4) | 261 (35.4) | 945 (37.4) | <0.001 |
| No | 1670 (58.6) | 477 (64.6) | 1583 (62.6) | |
| Physical activity *, n (% †) | ||||
| Yes | 254 (8.9) | 178 (24.2) | 384 (15.2) | <0.001 |
| No | 2610 (91.1) | 559 (75.8) | 2144 (84.8) | |
| Occupation *, n (% †) | ||||
| Indoor workers | 2737 (86.0) | 695 (84.1) | 2398 (86.5) | <0.001 |
| Outdoor workers | 252 (7.9) | 79 (9.6) | 208 (7.5) | |
| No jobs | 193 (6.1) | 52 (6.3) | 165 (6.0) | |
| Age Groups of Men (n = 7064) | |||||
| 10~29 (n = 1574) | 30~49 (n = 2548) | 50~69 (n = 2164) | ≥70 (n = 778) | p | |
| Age (years) | 20.0 (0.2) | 39.6 (0.2) | 57.7 (0.2) | 75.2 (0.2) | |
| Body weight (kg) | 66.1 (0.4) | 71.9 (0.3) | 67.7 (0.3) | 61.4 (0.4) | <0.001 |
| BMI (kg/m2) | 22.5 (0.1) | 24.4 (0.1) | 24.1 (0.1) | 22.8 (0.1) | <0.001 |
| Fat mass (kg) | 15.1 (0.2) | 16.2 (0.2) | 15.2 (0.2) | 14.3 (0.3) | <0.001 |
| Calcium intake (mg/d) | 539.8 (10.0) | 592.0 (8.0) | 579.1 (9.2) | 453.5 (14.5) | <0.001 |
| 25OHD (nmol/L) | 42.7 (0.5) | 47.9 (0.5) | 53.2 (0.7) | 52.4 (1.0) | <0.001 |
| Physical activity, n (%) | 123 (14.4) | 331 (13.0) | 293 (13.6) | 103 (13.4) | 0.162 |
| FNBMD (g/cm2) | 0.887 (0.005) | 0.840 (0.003) | 0.776 (0.003) | 0.674 (0.005) | <0.001 |
| LSBMD (g/cm2) | 0.939 (0.006) | 0.983 (0.003) | 0.955 (0.004) | 0.915 (0.008) | <0.001 |
| Geography *, n (%) | |||||
| North (37° N) | 832 (52.9) | 1296 (50.9) | 976 (45.1) | 325 (41.8) | |
| Middle (36° N) | 151 (9.6) | 267 (10.5) | 286 (13.2) | 120 (15.4) | |
| South (33° N~35° N) | 591 (37.5) | 985 (38.6) | 902 (41.7) | 333 (42.8) | |
| Age Groups of Women (n = 6779) | |||||
| 10~29 (n = 1554) | 30~49 (n = 2630) | 50~69 (n = 1747) | ≥70 (n = 848) | p | |
| Age (years) | 19.7 (0.2) | 39.6 (0.1) | 57.8 (0.2) | 76.1 (0.2) | |
| Body weight (kg) | 53.4 (0.3) | 58.0 (0.2) | 58.2 (0.2) | 53.3 (0.4) | <0.001 |
| BMI (kg/m2) | 20.9 (0.1) | 23.0 (0.1) | 24.5 (0.1) | 24.0 (0.1) | <0.001 |
| Fat mass (kg) | 17.5 (0.2) | 18.9 (0.1) | 20.1 (0.2) | 18.4 (0.3) | <0.001 |
| Calcium intake (mg/d) | 438.7 (8.2) | 469.2 (6.3) | 449.2 (9.1) | 327.0 (11.5) | <0.001 |
| 25OHD (nmol/L) | 38.9 (0.5) | 40.9 (0.5) | 44.9 (0.5) | 44.2 (0.7) | <0.001 |
| Physical activity, n (%) | 92 (9.8) | 325 (12.4) | 304 (17.6) | 95 (11.3) | <0.001 |
| FNBMD (g/cm2) | 0.763 (0.004) | 0.761 (0.003) | 0.665 (0.003) | 0.531 (0.004) | <0.001 |
| LSBMD (g/cm2) | 0.917 (0.004) | 0.996 (0.003) | 0.843 (0.004) | 0.730 (0.006) | <0.001 |
| Geography *, n (%) | |||||
| North (37° N) | 806 (51.9) | 1358 (51.6) | 714 (40.9) | 304 (35.8) | |
| Middle (36° N) | 192 (12.3) | 255 (9.7) | 248 (14.2) | 131 (15.5) | |
| South (33° N~36° N) | 556 (35.8) | 1017 (38.7) | 785 (44.9) | 413 (48.7) | |
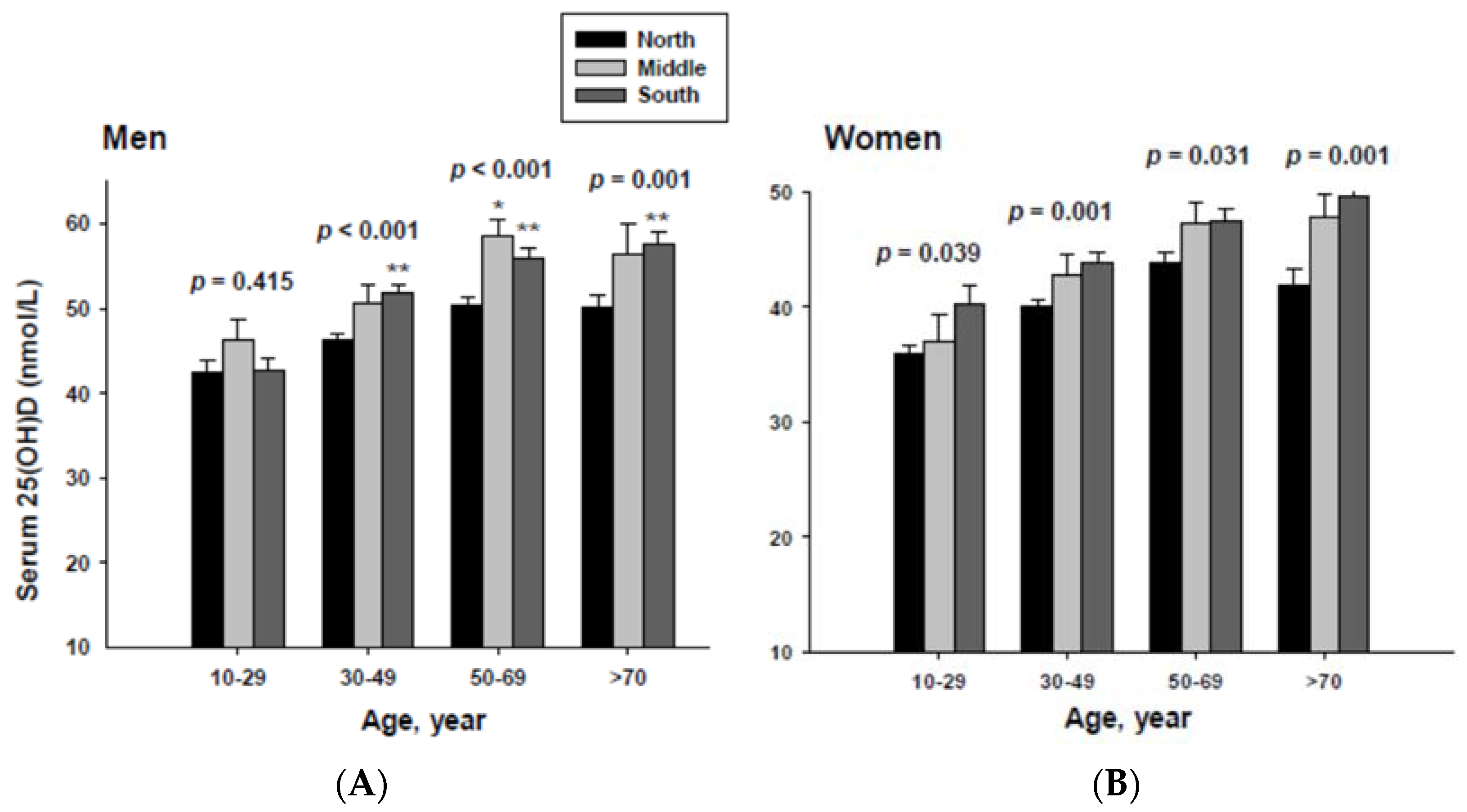
3.2. Serum 25(OH)D Concentrations Categorized by Geographic Location and Age
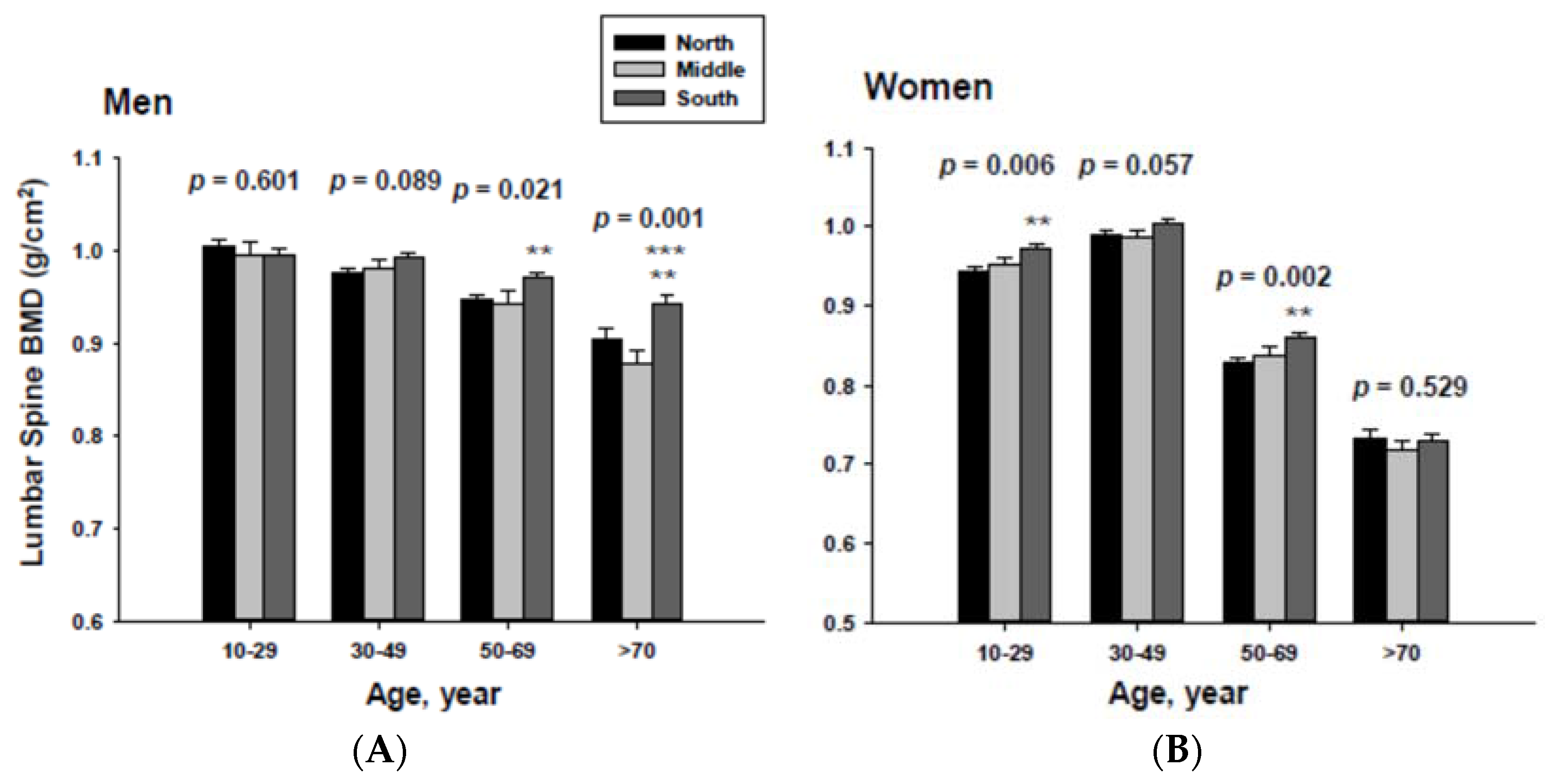
3.3. Bone Mineral Density Categorized by Geographic Location and Age

4. Discussion
5. Conclusions
Acknowledgments
Author contributions
Conflicts of interest
References
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V28–V33. [Google Scholar] [CrossRef]
- Lu, H.K.; Zhang, Z.; Ke, Y.H.; He, J.W.; Fu, W.Z.; Zhang, C.Q.; Zhang, Z.L. High prevalence of vitamin D insufficiency in China: Relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS ONE 2012, 7, e47264. [Google Scholar] [CrossRef] [PubMed]
- Ritu, D.; Gupta, A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients 2014, 6, 729–775. [Google Scholar]
- Kirii, K.; Mizoue, T.; Iso, H.; Takahashi, Y.; Kato, M.; Inoue, M.; Noda, M.; Tsugane, S. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia 2009, 52, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.K. Vitamin D insufficiency in Korea—A greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Gao, Y.T.; Dai, Q.; Yang, G.; Cai, H.; Li, H.; Zheng, W.; Shu, X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: The Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009, 89, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V. Dietary calcium intake—A critical reappraisal. Indian J. Med. Res. 2008, 127, 269–273. [Google Scholar] [PubMed]
- Joo, N.S.; Dawson-Hughes, B.; Kim, Y.S.; Oh, K.; Yeum, K.J. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: Analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHAENS IV-3, 2009 and V-1, 2010). J. Bone Miner. Res. 2013, 28, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.S.; Dawson-Hughes, B.; Yeum, K.J. 25-Hydroxyvitamin D, calcium intake, and bone mineral content in adolescents and young adults: Analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3, 2008-2009 and V-1, 2010). J. Clin. Endocrinol. Metab. 2013, 98, 3627–3636. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, K.; Ko, Y.J.; Kim, S.J.; Oh, S.I.; Durrance, D.Y.; Yoo, D.; Park, S.M. Prevalence, awareness, and treatment of osteoporosis among Korean women: The Fourth Korea National Health and Nutrition Examination Survey. Bone 2012, 50, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Siris, E.S.; Binkley, N.; Beard, M.K.; Khan, A.; Katzer, J.T.; Petruschke, R.A.; Chen, E.; de Papp, A.E. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J. Clin. Endocrinol. Metab. 2005, 90, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Hirai, C.; Nakajima, H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J. Nutr. Sci. Vitaminol. 2013, 59, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hagenau, T.; Vest, R.; Gissel, T.N.; Poulsen, C.S.; Erlandsen, M.; Mosekilde, L.; Vestergaard, P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos. Int. 2009, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Lucas, R.M.; Harrison, S.L.; van der Mei, I.; Armstrong, B.K.; Whiteman, D.C.; Kricker, A.; Nowak, M.; Brodie, A.M.; Sun, J. The contributions of solar ultraviolet radiation exposure and other determinants to serum 25-hydroxyvitamin D concentrations in Australian adults: The AusD Study. Am. J. Epidemiol. 2014, 179, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Klassen, K.M.; Fairley, C.K.; Kimlin, M.G.; Kelly, M.; Read, T.R.; Broom, J.; Russell, D.B.; Ebeling, P.R. Ultraviolet index and location are important determinants of vitamin D status in people with human immunodeficiency virus. Photochem. Photobiol. 2015, 91, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin, D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Brockwell, S.E.; Mehta, V.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.; Cauley, J.A.; Danielson, M.E.; et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.T.; Lee, C.M.; Park, J.H.; Ko, J.A.; Seong, E.J.; Park, M.S.; Cho, B. Milk intake and its association with metabolic syndrome in Korean: Analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). J. Korean Med. Sci. 2010, 25, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, B.K. Vitamin D deficiency is an independent risk factor for cardiovascular disease in Koreans aged >/= 50 years: Results from the Korean National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2012, 6, 162–168. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeum, K.-J.; Song, B.C.; Joo, N.-S. Impact of Geographic Location on Vitamin D Status and Bone Mineral Density. Int. J. Environ. Res. Public Health 2016, 13, 184. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13020184
Yeum K-J, Song BC, Joo N-S. Impact of Geographic Location on Vitamin D Status and Bone Mineral Density. International Journal of Environmental Research and Public Health. 2016; 13(2):184. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13020184
Chicago/Turabian StyleYeum, Kyung-Jin, Byeng Chun Song, and Nam-Seok Joo. 2016. "Impact of Geographic Location on Vitamin D Status and Bone Mineral Density" International Journal of Environmental Research and Public Health 13, no. 2: 184. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13020184





