Determination of Sediment Oxygen Demand in the Ziya River Watershed, China: Based on Laboratory Core Incubation and Microelectrode Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Core Incubation Measurements
2.4. Microelectrode Measurements
3. Results and Discussion
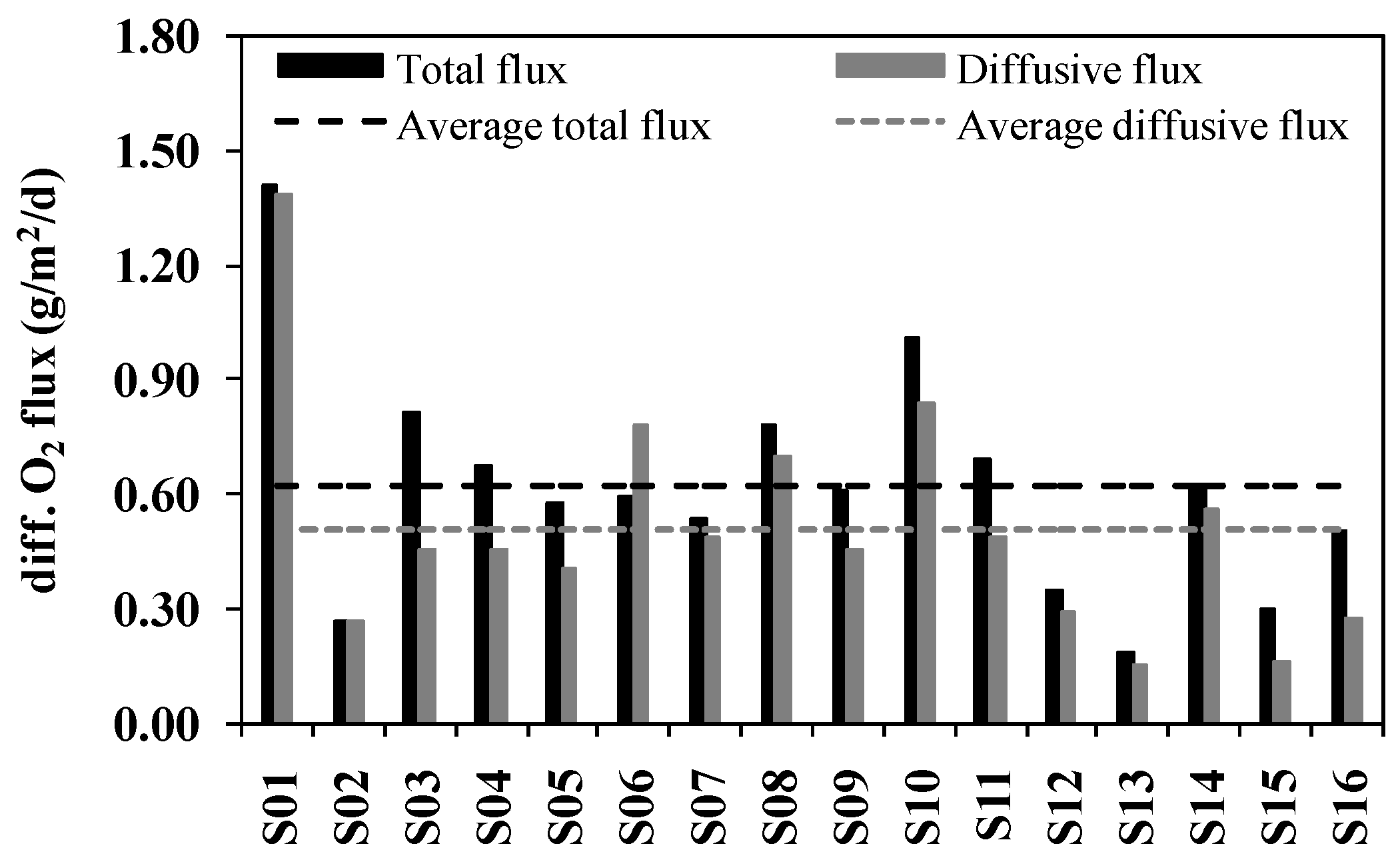
3.1. SOD Determined by Core Incubations
3.2. SOD Determined by Microelectrodes
3.3. Comparison between Incubations and Microelectrodes
3.4. Effects of Point and Non-Point Pollutions on SOD
3.5. Effects of SOD on the River System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cox, B. A review of dissolved oxygen modelling techniques for lowland rivers. Sci. Total Environ. 2003, 314, 303–334. [Google Scholar] [CrossRef]
- Hladyz, S.; Watkins, S.C.; Whitworth, K.L.; Baldwin, D.S. Flows and hypoxic blackwater events in managed ephemeral river channels. J. Hydrol. 2011, 401, 117–125. [Google Scholar] [CrossRef]
- Veenstra, J.N.; Nolen, S.L. In-situ sediment oxygen demand in five Southwestern US lakes. Water Res. 1991, 25, 351–354. [Google Scholar] [CrossRef]
- Wang, W. Kinetics of sediment oxygen demand. Water Res. 1981, 15, 475–482. [Google Scholar] [CrossRef]
- Hanes, N.; Irvine, R.L. Oxygen Uptake Rates of Benthal Systems by A New Technique. Avilable online: http://earchives.lib.purdue.edu/cdm/ref/collection/engext/id/13592 (accessed on 14 January 2016).
- James, A. The measurement of benthal respiration. Water Res. 1974, 8, 955–959. [Google Scholar] [CrossRef]
- Burns, N.; Ross, C. Oxygen-nutrient relationships within the central basin of Lake Erie. In Nutrients in Natural Waters; Allen, H.E., Kramer, J.R., Eds.; John Wiley & Sons: New York, NY, USA, 1972. [Google Scholar]
- Matlock, M.D.; Kasprzak, K.R.; Osborn, G.S. Sediment Oxygen Demand in the Arroyo Colorado River. J. Am. Water Resour. Assoc. 2003, 39, 267–275. [Google Scholar] [CrossRef]
- Chau, K. Field measurements of sod and sediment nutrient fluxes in a land-locked embayment in hong kong. Adv. Environ. Res. 2002, 6, 135–142. [Google Scholar] [CrossRef]
- Bowman, G.T.; Delfino, J.J. Sediment oxygen demand techniques: A review and comparison of laboratory and in situ systems. Water Res. 1980, 14, 491–499. [Google Scholar] [CrossRef]
- Boudreau, B.P.; Jørgensen, B. Diagenesis and Sediment-Water Exchange; Oxford University Press: New York, NY, USA, 2001; pp. 211–244. [Google Scholar]
- Wenzhöfer, F.; Glud, R.N. Small-scale spatial and temporal variability in coastal benthic O2 dynamics: Effects of fauna activity. Limnol. Oceanogr. 2004, 49, 1471–1481. [Google Scholar] [CrossRef]
- De Beer, D.; Wenzhöfer, F.; Ferdelman, T.G.; Boehme, S.E.; Huettel, M.; van Beusekom, J.E.; Böttcher, M.E.; Musat, N.; Dubilier, N. Transport and mineralization rates in North Sea sandy intertidal sediments, Sylt-Rømø Basin, Wadden Sea. Limnol. Oceanogr. 2005, 50, 113–127. [Google Scholar] [CrossRef]
- Archer, D.; Devol, A. Benthic oxygen fluxes on the washington shelf and slope: A comparison of in situ microelectrode and chamber flux measurements. Limnol. Oceanogr. 1992, 37, 614–629. [Google Scholar] [CrossRef]
- Lindeboom, H.J.; Sandee, A.J.; Driessche, H.A. A new bell jar/microelectrode method to measure changing oxygen fluxes in illuminated sediments with a microalgal cover1. Limnol. Oceanogr. 1985, 30, 693–698. [Google Scholar] [CrossRef]
- Reimers, C.E.; Jahnke, R.A.; McCorkle, D.C. Carbon fluxes and burial rates over the continental slope and rise off central california with implications for the global carbon cycle. Glob. Biogeochem. Cycles 1992, 6, 199–224. [Google Scholar] [CrossRef]
- Hickey, C.W. Benthic chamber for use in rivers: Testing against oxygen mass balances. J. Environ. Eng. 1988, 114, 828–845. [Google Scholar] [CrossRef]
- Seiki, T.; Izawa, H.; Date, E.; Sunahara, H. Sediment oxygen demand in hiroshima bay. Water Res. 1994, 28, 385–393. [Google Scholar] [CrossRef]
- Baity, H.G. Some factors affecting the aerobic decomposition of sewage sludge deposits. Sewage Works J. 1938, 10, 539–568. [Google Scholar]
- Thomann, R.V.; Mueller, J.A. Principles of Surface Water Quality Modeling and Control; Harper & Row: New York, NY, USA, 1987. [Google Scholar]
- Wang, C.; Shan, B.; Zhang, H.; Rong, N. Analyzing sediment dissolved oxygen based on microprofile modeling. Environ. Sci. Poll. Res. 2014, 21, 10320–10328. [Google Scholar] [CrossRef] [PubMed]
- Sweerts, J.P.R.; Bär-Gilissen, M.J.; Cornelese, A.A.; Cappenberg, T.E. Oxygen-consuming processes at the profundal and littoral sediment-water interface of a small meso-eutrophic lake (Lake Vechten, The Netherlands). Limnol. Oceanogr. 1991, 36, 1124–1133. [Google Scholar] [CrossRef]
- Bryant, L.D.; McGinnis, D.F.; Lorrai, C.; Brand, A.; Little, J.C.; Wüest, A. Evaluating oxygen fluxes using microprofiles from both sides of the sediment-water interface. Limnol. Oceanogr. Methods 2010, 8, 610–627. [Google Scholar] [CrossRef]
- House, W.A. Factors influencing the extent and development of the oxic zone in sediments. Biogeochemistry 2003, 63, 317–334. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Revsbech, N.P. Diffusive boundary layers and the oxygen uptake of sediments and detritus1. Limnol. Oceanogr. 1985, 30, 111–122. [Google Scholar] [CrossRef]
- Rasmussen, H.; Jørgensen, B.B. Microelectrode studies of seasonal oxygen uptake in a coastal sediment: Role of molecular diffusion. Marine Ecol. Progr. Series 1992, 81, 289–303. [Google Scholar] [CrossRef]
- Steinberger, N.; Hondzo, M. Diffusional mass transfer at sediment-water interface. J. Environ. Eng. 1999, 125, 192–200. [Google Scholar] [CrossRef]
- Rabouille, C.; Denis, L.; Dedieu, K.; Stora, G.; Lansard, B.; Grenz, C. Oxygen demand in coastal marine sediments: Comparing in situ microelectrodes and laboratory core incubations. J. Exp. Marine Biol. Ecol. 2003, 285, 49–69. [Google Scholar] [CrossRef]
- Dedieu, K.; Rabouille, C.; Thouzeau, G.; Jean, F.; Chauvaud, L.; Clavier, J.; Mesnage, V.; Ogier, S. Benthic O2 distribution and dynamics in a mediterranean lagoon (Thau, France): An in situ microelectrode study. Estuar. Coast. Shelf Sci. 2007, 72, 393–405. [Google Scholar] [CrossRef]
- Zhang, P.; Pang, Y.; Pan, H.; Shi, C.; Huang, Y.; Wang, J. Factors contributing to hypoxia in the Minjiang river estuary, Southeast China. Int. J. Environ. Res. Public Health 2015, 12, 9357–9374. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.C.L.; Pei, L.; Baoqing, S.; Yu, Z. Evaluation of river eutrophication of the haihe river basin. Acta Sci. Circumstantiae 2015, 35, 2336–2344. (In Chinese) [Google Scholar]
- Hong, Z.C.L.; Pei, L.; Baoqing, S.; Yu, Z. Trends of pollution by the oxygen-consuming substances and the distribution of oxygen deficiency in the haihe river basin. Acta Sci. Circumstantiae 2015, 35, 2324–2335. (In Chinese) [Google Scholar]
- Sommaruga, R. Sediment oxygen demand in man-made Lake Ton-Ton (Uruguay). Hydrobiologia 1991, 215, 215–221. [Google Scholar] [CrossRef]
- Kemp, W.M.; Sampou, P.; Garber, J.; Tuttle, J.; Boynton, W. Seasonal depletion of oxygen from bottom waters of chesapeake bay: Roles of benthic and planktonic respiration and physical exchange processes. Marine Ecol. Progr. Series 1992, 85, 137–152. [Google Scholar] [CrossRef]
- Liu, W.-C.; Chen, W.-B.; Kimura, N. Measurement of sediment oxygen demand to simulate dissolved oxygen distribution: Case study in the main Danshuei river estuary. Environ. Eng. Sci. 2009, 26, 1701–1711. [Google Scholar] [CrossRef]
- Liu, W.-C.; Chen, W.-B. Monitoring sediment oxygen demand for assessment of dissolved oxygen distribution in river. Environ. Monit. Assess. 2012, 184, 5589–5599. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; McEachern, P.; Yu, T.; Zhu, D.Z. Model development for prediction and mitigation of dissolved oxygen sags in the Athabasca River, Canada. Sci. Total Environ. 2013, 443, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, Q.; Shao, S.; Zhang, N.; Shen, Q.; Liu, C. Heavy metal pollution, fractionation, and potential ecological risks in sediments from Lake Chaohu (Eastern China) and the surrounding rivers. Int. J. Environ. Res. Public Health 2015, 12, 14115–14131. [Google Scholar] [CrossRef] [PubMed]
- Gunnison, D.; Chen, R.; Brannon, J. Relationship of materials in flooded soils and sediments to the water quality of reservoirs—I: Oxygen consumption rates. Water Res. 1983, 17, 1609–1617. [Google Scholar] [CrossRef]
- Brannon, J.; Gunnison, D.; Smart, R.; Chen, R. Effects of added organic matter on iron and manganese redox systems in sediment. Geomicrobiol. J. 1984, 3, 319–341. [Google Scholar] [CrossRef]




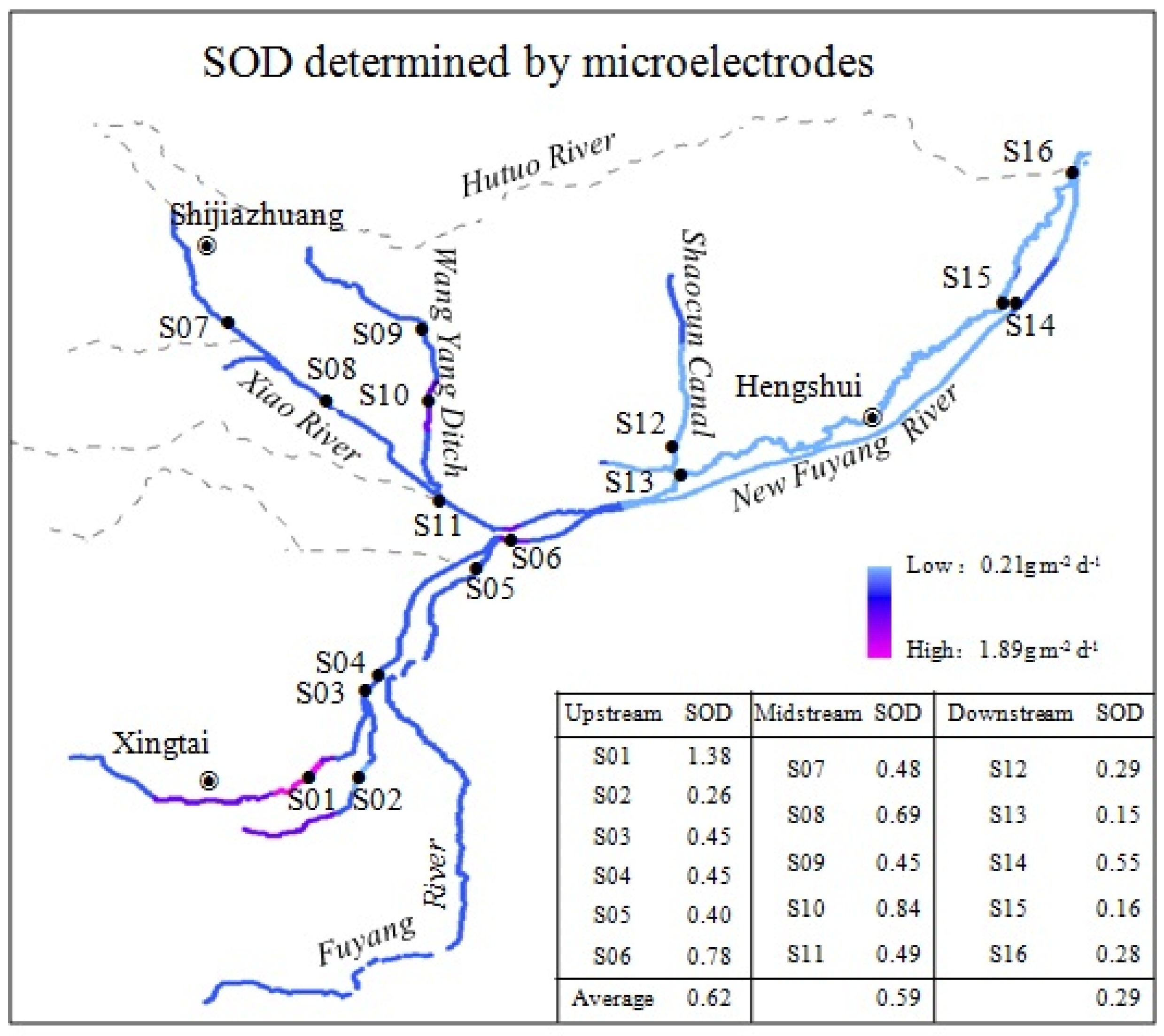

| River | Sample Site | Pollutants Quantity Inlets Into Rivers | Contributions of Industrial Pollution Sources | |||
|---|---|---|---|---|---|---|
| COD (103 t) | Ammonia (t) | COD | Ammonia | |||
| Upstream | Shunshui River | S01 | 33.00 | 0.16 | 65% | 21% |
| South Li River | S02 | |||||
| Li River | S03–S05 | |||||
| Midstream | Xiao River | S07, S08 | 39.00 | 0.48 | 42% | 33% |
| Wang Yang Ditch | S09, S10 | 2.30 | 0.15 | ~100% | ~100% | |
| Downstream | Shaocun Canal | S12, S13 | 4.50 | 0.52 | ~100% | ~100% |
| Fuyang River | S14–S16 | 34.00 | 2.03 | 30% | 20% | |
| TN | NH3-N | NO3−-N | NO2−-N | TP | SRP | SOD | COD | |
|---|---|---|---|---|---|---|---|---|
| TN | 1.000 | 0.915 ** | 0.291 | 0.249 | 0.382 | 0.225 | 0.350 | 0.333 |
| NH3-N | 1.000 | 0.079 | 0.124 | 0.285 | 0.219 | 0.006 | 0.056 | |
| NO3−-N | 1.000 | 0.411 | 0.253 | −0.118 | 0.441 | 0.212 | ||
| NO2−-N | 1.000 | 0.003 | −0.310 | 0.182 | 0.246 | |||
| TP | 1.000 | 0.770 ** | 0.837 ** | 0.077 | ||||
| SRP | 1.000 | 0.721 ** | 0.174 | |||||
| SOD | 1.000 | 0.642 ** | ||||||
| COD | 1.000 |
| Pollution Condition | Range of SOD at 20°C (g/(m2·d)) | Location | References |
|---|---|---|---|
| Admittingabundantdomestic wastewater, severe eutrophication | 0.48–1.44 | man-madeLake Ton-Ton, Uruguay | [33] |
| summertime occurrences of anoxia/hypoxia | 0–1.64 | Chesapeake Bay, USA | [34] |
| Low DO, ~4 mg/L | 0.47–1.28 | Tolo Harbor, Hong Kong | [9] |
| municipal andagricultural wastewater, BOD: 2–5 mg/L | 0.13–1.36 | Arroyo Colorado River | [8] |
| urban sewage and industrial wastewater pollution, hypoxia | 0.24–1.58 | Keelung River, Taiwan | [35] |
| Low DO (less than 1 mg/L), very high nutrient concentrations in the river | 0.37–1.25 | Xindian River, Taiwan | [36] |
| admitting paper industry wastewater | 0.22–1.82 | Athabasca River | [37] |
| Low DO, ~3 mg/L, admitting abundant domestic and industrial wastewater, severe hypoxia | 0.19–1.41 | the Ziya River Watershed, China | This study |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, N.; Shan, B.; Wang, C. Determination of Sediment Oxygen Demand in the Ziya River Watershed, China: Based on Laboratory Core Incubation and Microelectrode Measurements. Int. J. Environ. Res. Public Health 2016, 13, 232. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13020232
Rong N, Shan B, Wang C. Determination of Sediment Oxygen Demand in the Ziya River Watershed, China: Based on Laboratory Core Incubation and Microelectrode Measurements. International Journal of Environmental Research and Public Health. 2016; 13(2):232. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13020232
Chicago/Turabian StyleRong, Nan, Baoqing Shan, and Chao Wang. 2016. "Determination of Sediment Oxygen Demand in the Ziya River Watershed, China: Based on Laboratory Core Incubation and Microelectrode Measurements" International Journal of Environmental Research and Public Health 13, no. 2: 232. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13020232





