Salivary Alpha-Amylase Reactivity in Breast Cancer Survivors
Abstract
:1. Introduction
1.1. Salivary Cortisol as a Stress Biomarker
1.2. Salivary Alpha-Amylase as a Stress Biomarker
2. Method
2.1. Participants
2.2. Measures of Stress
2.2.1. Salivary Alpha-Amylase
2.2.2. Trier Social Stress Test (TSST)
2.2.3. Visual Analog Scale (VAS)
2.2.4. Questionnaires
2.3. Procedure
3. Results
3.1. Participant Characteristics
3.2. Data Analysis
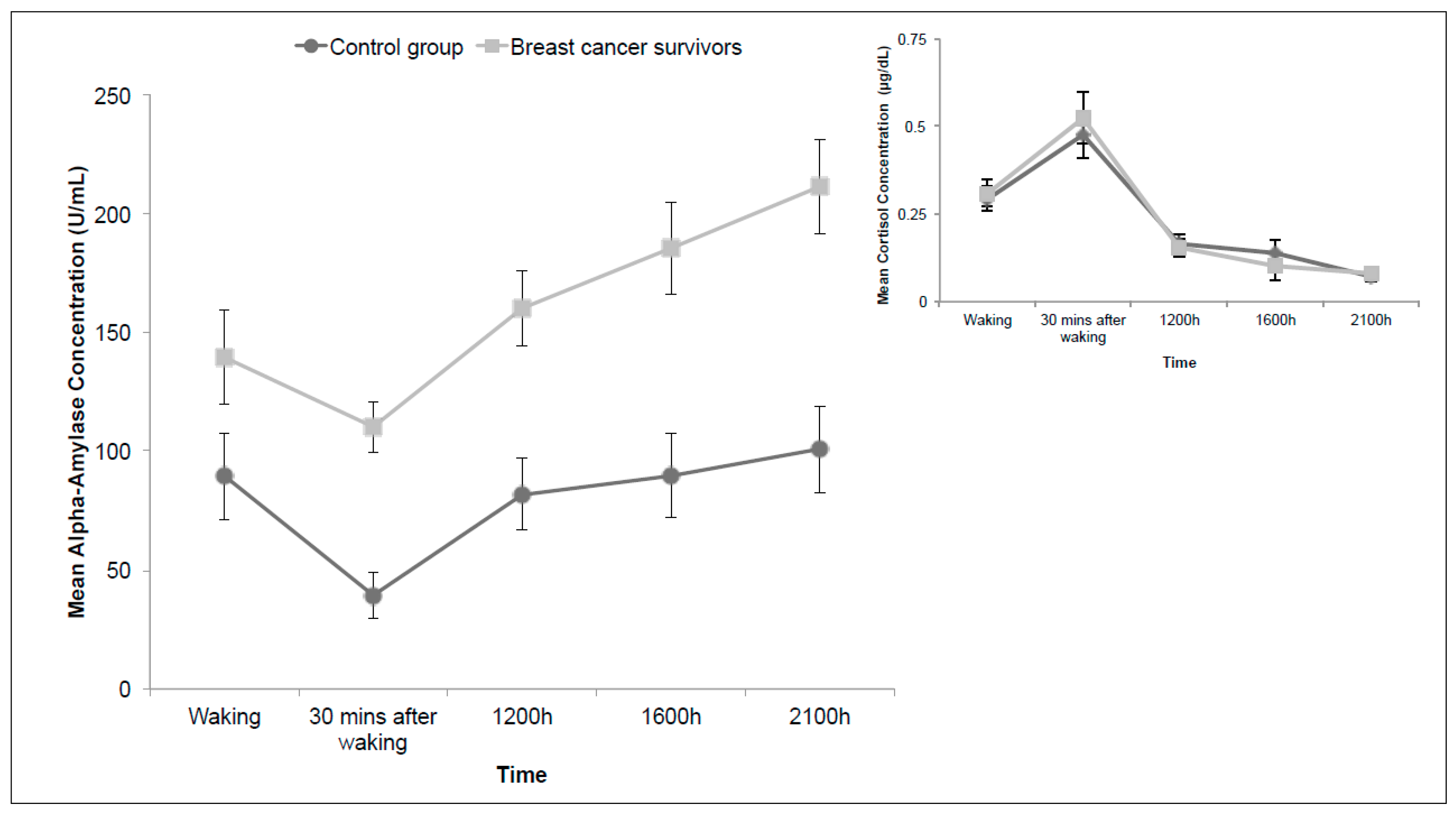
3.3. Diurnal Alpha-Amylase
3.4. Alpha-Amylase in Response to Acute Stress
3.5. Area Under the Curve: Alpha-Amylase
3.6. Subjective Measures in Relation to Alpha-Amylase
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canadian Cancer Society. Breast Cancer Statistics; Canadian Cancer Society: Toronto, ON, Canada, 2015. [Google Scholar]
- Lundberg, U. Stress hormones in health and illness: The roles of work and gender. Psychoneuroendocrinology 2005, 30, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, A.; Koch, U. Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: A prospective study. Psychooncology 2007, 16, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Knobf, M.T. Psychosocial responses in breast cancer survivors. Semin. Oncol. Nurs. 2011, 27, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Kedde, H.; Van de Wiel, H.; Schultz, W.W.; Wijsen, C. Sexual dysfunction in young women with breast cancer. Support. Care Cancer 2013, 21, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Tiedtke, C.; de Rijk, A.; Dierckx de Casterlé, B.; Christiaens, M.; Donceel, P. Experiences and concerns about “returning to work” for women breast cancer survivors: A literature review. Psycho-Oncology 2010, 19, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Hauken, M.A.; Larsen, T.M.; Holsen, I. Meeting reality: Young adult cancer survivors’ experiences of reentering everyday life after cancer treatment. Cancer Nurs. 2013, 36, E17–E26. [Google Scholar] [CrossRef] [PubMed]
- Thiel, K.J.; Dretsch, M.N. The basics of the stress response: A historical context and introduction. In The Handbook of Stress: Neuropsychological Effects on the Brain; Conrad, C.D., Ed.; Wiley-Blackwell: Oxford, UK, 2011; pp. 1–28. [Google Scholar]
- Strahler, J.; Mueller, A.; Rosenloecher, F.; Kirschbaum, C.; Rohleder, N. Salivary α-amylase stress reactivity across different age groups. Psychophysiology 2010, 47, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Couture-Lalande, M.; Lebel, S.; Bielajew, C. Analysis of the cortisol diurnal rhythmicity and cortisol reactivity in long-term breast cancer survivors. Breast Cancer Manag. 2014, 3, 465–476. [Google Scholar] [CrossRef]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.A.; Schwartz, J.E.; Smyth, J.; Kirschbaum, C.; Cohen, S.; Hellhammer, D.; Grossman, S. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology 2001, 26, 295–306. [Google Scholar] [CrossRef]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.; Kirschbaum, C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010, 35, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Armario, A.; Vallès, A.; Dal-Zotto, S.; Márquez, C.; Belda, X. A single exposure to severe stressors causes long-term desensitisation of the physiological response to the homotypic stressor. Stress Int. J. Biol. Stress 2004, 7, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, H.C.; Giese-Davis, J.; Sephton, S.; Epel, E.S.; Turner-Cobb, J.M.; Spiegel, D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology 2004, 29, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.S.; Mishel, M.; Neelon, V.; Belyea, M.; Pisano, E.; Soo, M.S. Cortisol levels and responses to mammography screening in breast cancer survivors: A pilot study. Psychosom. Med. 2003, 65, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.; Giese-Davis, J.; Taylor, C.B.; Kraemer, H. Stress sensitivity in metastatic breast cancer: Analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 2006, 31, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Bogdan, A.; Levi, F.; Benavides, M.; Auzeby, A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br. J. Cancer 1996, 74, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Sephton, S.E.; Sapolsky, R.M.; Kraemer, H.C.; Spiegel, D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000, 92, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Batzri, S.; Selinger, Z. Enzyme secretion mediated by the epinephrine-receptor in rat parotid slices. Factors governing efficiency of the process. J. Biol. Chem. 1973, 248, 356–360. [Google Scholar] [PubMed]
- Batzri, S.; Selinger, Z.; Schramm, M.; Robinovitch, M.R. Potassium release mediated by the epinephrine-receptor in rat parotid slices. Properties and relation to enzyme secretion. J. Biol. Chem. 1973, 248, 361–368. [Google Scholar] [PubMed]
- Anderson, L.; Garrett, J.; Johnson, D.; Kauffman, D.; Keller, P.; Thulin, A. Influence of circulating catecholamines on protein secretion into rat parotid saliva during parasympathetic stimulation. J. Physiol. (Lond.) 1984, 352, 163–171. [Google Scholar] [CrossRef]
- Asking, B. Sympathetic stimulation of amylase secretion during a parasympathetic background activity in the rat parotid gland. Acta Physiol. Scand. 1985, 124, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Speirs, R.; Herring, J.; Cooper, W.; Hardy, C.; Hind, C. The Influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Arch. Oral Biol. 1974, 19, 747–752. [Google Scholar] [CrossRef]
- Granger, D.A.; Kivlighan, K.T.; El-Shiekh, M.; Gordis, E.B.; Stroud, L.R. Salivary α-amylase in biobehavioral research. Ann. N. Y. Acad. Sci. 2007, 1098, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.A.; Veerman, E.C.; de Geus, E.J.; Proctor, G.B. α-Amylase as a reliable and convenient measure of sympathetic activity: Don’t start salivating just yet! Psychoneuroendocrinology 2011, 36, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.T.; Vogelsong, K.M.; Lu, Y.; Ellman, A.B.; Hudgens, G.A. Salivary α-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996, 16, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Thornton, R.; Miller, D.; Biersner, R. Effects of exercise stress on parotid gland secretion. Horm. Metab. Res. 1979, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Nater, U.M. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology 2009, 34, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.A.; Brand, H.; Ligtenberg, A.; Bermond, B.; Hoogstraten, J.; Nieuw-Amgerongen, A. The response of salivary protein levels and S-IgA to an academic examination are associated with daily stress. J. Psychophysiol. 1998, 12, 384–391. [Google Scholar]
- Chatterton, R.T., Jr.; Vogelsong, K.M.; Lu, Y.; Hudgens, G.A. Hormonal responses to psychological stress in men preparing for skydiving 1. J. Clin. Endocrinol. Metab. 1997, 82, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Skosnik, P.D.; Chatterton, R.T.; Swisher, T.; Park, S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. Int. J. Psychophysiol. 2000, 36, 59–68. [Google Scholar] [CrossRef]
- Allen, A.P.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Biological and psychological markers of stress in humans: Focus on the trier social stress test. Neurosci. Biobehav. Rev. 2014, 38, 94–124. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The “trier social stress test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Hellhammer, D.H.; Kirschbaum, C. Ten years of research with the trier social stress test—revisited. In Social Neuroscience; Harmon-Jones, E., Winkielman, P., Eds.; The Guilford Press: New York, NY, USA, 2007; pp. 56–83. [Google Scholar]
- Gordis, E.B.; Granger, D.A.; Susman, E.J.; Trickett, P.K. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm. Behav. 2008, 53, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C.; Duncko, R.; Covington, M.F.; Kopperman, L.; Kling, M.A. Acute stress potentiates anxiety in humans. Biol. Psychiatry 2007, 62, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Salimetrics. Salivary α-Amylase Kinetic Enzyme Assay Kit; Salimetrics: Carlsbad, CA, USA, 2015. [Google Scholar]
- Aitken, R.C. Measurement of feelings using visual analogue scales. Proc. R. Soc. Med. 1969, 62, 989–993. [Google Scholar] [PubMed]
- Brantley, P.J.; Waggoner, C.D.; Jones, G.N.; Rappaport, N.B. A daily stress inventory: Development, reliability, and validity. J. Behav. Med. 1987, 10, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Sarason, I.G.; Johnson, J.H.; Siegel, J.M. Assessing the impact of life changes: Development of the life experiences survey. J. Consult. Clin. Psychol. 1978, 46, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Vickberg, S.M. The concerns about recurrence scale (CARS): A systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann. Behav. Med. 2003, 25, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2013. [Google Scholar]
- Nater, U.M.; Rohleder, N.; Schlotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human Salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P.; Eyer, J. Allostasis: A New Paradigm to Explain Arousal Pathology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1988. [Google Scholar]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [PubMed]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom. Med. 2003, 65, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Lebel, S.; Rosberger, Z.; Edgar, L.; Devins, G.M. Predicting stress-related problems in long-term breast cancer survivors. J. Psychosom. Res. 2008, 65, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lebel, S.; Rosberger, Z.; Edgar, L.; Devins, G.M. Comparison of four common stressors across the breast cancer trajectory. J. Psychosom. Res. 2007, 63, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Silverstein, K.; Arès, I.; Bielajew, C. The factors that influence fear of recurrence in breast cancer survivors. in preparation.
- Strahler, J.; Berndt, C.; Kirschbaum, C.; Rohleder, N. Aging diurnal rhythms and chronic stress: Distinct alteration of diurnal rhythmicity of salivary α-amylase and cortisol. Biol. Psychol. 2010, 84, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Dinenno, F.A. Collateral damage: Cardiovascular consequences of chronic sympathetic activation with human aging. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1895–H1905. [Google Scholar] [CrossRef] [PubMed]
- Almela, M.; Hidalgo, V.; Villada, C.; van der Meij, L.; Espín, L.; Gómez-Amor, J.; Salvador, A. Salivary alpha-amylase response to acute psychosocial stress: The impact of age. Biol. Psychol. 2011, 87, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.M.; Nicholls, E.; Chen, E. Chronic stress, salivary cortisol, and α-amylase in children with asthma and healthy children. Biol. Psychol. 2008, 78, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.V.; Joksimovic, L.; Kirschbaum, C.; Wolf, J.M.; Rohleder, N. Altered salivary alpha-amylase awakening response in bosnian war refugees with posttraumatic stress disorder. Psychoneuroendocrinology 2012, 37, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Yigla, M.; Berkovich, Y.; Nagler, R.M. Oxidative stress indices in COPD—Broncho-alveolar lavage and salivary analysis. Arch. Oral Biol. 2007, 52, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Tumilasci, O.R.; Cersosimo, M.; Belforte, J.E.; Micheli, F.E.; Benarroch, E.E.; Pazo, J.H. Quantitative study of salivary secretion in parkinson’s disease. Mov. Disord. 2006, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. BMB Rep. 2007, 40, 29–35. [Google Scholar] [CrossRef]
- Rohleder, N.; Marin, T.J.; Ma, R.; Miller, G.E. Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. J. Clin. Oncol. 2009, 27, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, D.L.; Kuhn, R.; Kinney, A.Y.; Donaldson, G.W.; Nakamura, Y. Reduction in salivary α-amylase levels following a mind–body intervention in cancer survivors—An exploratory study. Psychoneuroendocrinology 2013, 38, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Ariza-García, A.; Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Arroyo-Morales, M. Influence of physical inactivity in psychophysiolocigal state of breast cancer survivors. Eur. J. Cancer Care 2013, 22, 738–745. [Google Scholar] [CrossRef] [PubMed]


| Demographic Characteristics | Breast Cancer Survivors (N = 22) | Control Group (N = 26) |
|---|---|---|
| Age (years) mean ± SD | 58.9 ± 10.1 | 57.4 ± 11 |
| No. of Participants (%) | No. of Participants (%) | |
| Ethnicity | ||
| White | 20 (90.9) | 23 (88.5) |
| Black | 1 (3.8) | |
| Asian | 2 (7.7) | |
| First Nations | 2 (9.1) | |
| Highest level of education | ||
| High School | 6 (27.3) | 9 (34.6) |
| College | 4 (18.2) | 4 (15.4) |
| Bachelor’s degree | 11 (50) | 7 (26.9) |
| Master’s degree | 1 (4.5) | 5 (19.2) |
| Doctoral degree | 1 (3.8) | |
| Family income (CDN) * | ||
| Under $40,000 | 3 (15) | 5 (20.8) |
| $40,000 to $79,999 | 10 (50) | 10 (41.7) |
| $80,000 to $ 119,999 | 5 (25) | 5 (20.8) |
| $120,000 and over | 2 (10) | 4 (16.7) |
| Medical Characteristics | Breast Cancer Survivors (N = 22) |
|---|---|
| Mean age of diagnosis ± SD (years) | 54.1 ± 8.7 |
| Mean time (years) since diagnosis ± SD (years) | 4.6 ± 3 |
| Breast cancer stage | No. of Participants (%) |
| 0 | 4 (18.2) |
| 1 | 10 (45.5) |
| 2 | 5 (22.7) |
| 3 | 2 (13.6) |
| Type of surgery | |
| Unilateral mastectomy | 6 (27.3) |
| Bilateral mastectomy | 7 (31.8) |
| Lumpectomy | 9 (40.9) |
| Treatment * | |
| Chemotherapy | 10 (45.5) |
| Hormone therapy | 14 (63.6) |
| Radiation therapy | 14 (63.6) |
| Breast cancer recurrence | |
| None | 20 (83.3) |
| One recurrence | 1 (4.2) |
| Two recurrences | 1 (4.2) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Couture-Lalande, M.-È.; Narain, T.A.; Lebel, S.; Bielajew, C. Salivary Alpha-Amylase Reactivity in Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2016, 13, 353. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13040353
Wan C, Couture-Lalande M-È, Narain TA, Lebel S, Bielajew C. Salivary Alpha-Amylase Reactivity in Breast Cancer Survivors. International Journal of Environmental Research and Public Health. 2016; 13(4):353. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13040353
Chicago/Turabian StyleWan, Cynthia, Marie-Ève Couture-Lalande, Tasha A. Narain, Sophie Lebel, and Catherine Bielajew. 2016. "Salivary Alpha-Amylase Reactivity in Breast Cancer Survivors" International Journal of Environmental Research and Public Health 13, no. 4: 353. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13040353






