Concurrent Phosphorus Recovery and Energy Generation in Mediator-Less Dual Chamber Microbial Fuel Cells: Mechanisms and Influencing Factors
Abstract
:1. Introduction
2. Materials and Methods
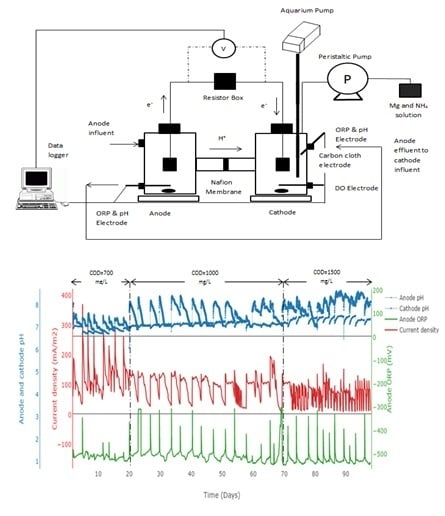
2.1. MFC Configuration
2.2. MFC Experimental Design and Start-Up
2.3. Analytical Methods
2.4. Phosphorus Precipitation in MFC
2.5. Statistical Analyses
3. Results and Discussion
3.1. Phosphorus Recovery and Electricity Generation in Dual Chamber MFC
3.2. COD Removal and Coulombic Efficiency
3.3. Effect of Different Operational Parameters
3.3.1. P recovery at Different Substrate Concentrations
3.3.2. P Recovery at Different Anode and Cathode Volumes
3.3.3. P Recovery at Different Aeration Flow Rates at the Cathode
3.4. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Güney, K.; Weidelener, A.; Krampe, J. Phosphorus recovery from digested sewage sludge as map by the help of metal ion separation. Water Res. 2008, 42, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- You, J.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Electricity generation and struvite recovery from human urine using microbial fuel cells. J. Chem. Technol. Biotechnol. 2015, 91, 647–654. [Google Scholar] [CrossRef]
- Cusick, R.D.; Ullery, M.L.; Dempsey, B.A.; Logan, B.E. Electrochemical struvite precipitation from digestate with a fluidized bed cathode microbial electrolysis cell. Water Res. 2014, 54, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, Y.; Clark, T.; Pearce, P.; Parsons, S. Potential phosphorus recovery by struvite formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Cusick, R.D.; Logan, B.E. Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 2012, 107, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Ichihashi, O. Phosphorus recovery from artificial wastewater by microbial fuel cell and its effect on power generation. Bioresour. Technol. 2013, 137, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.; Darus, L.; Hamelers, H.V.; Buisman, C.J. Effect of operational parameters on coulombic efficiency in bioelectrochemical systems. Bioresour. Technol. 2011, 102, 11172–11176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour. Technol. 2011, 102, 4468–4473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, Z. Simultaneous nitrification and denitrification with electricity generation in dual-cathode microbial fuel cells. J. Chem. Technol. Biotechnol. 2012, 87, 153–159. [Google Scholar] [CrossRef]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.; Ghangrekar, M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Kim, H.W.; Lim, K.H.; Shin, H.S. Effects of organic loading rates on the continuous electricity generation from fermented wastewater using a single-chamber microbial fuel cell. Bioresour. Technol. 2010, 101, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Mashkour, M.; Rahimnejad, M. Effect of various carbon-based cathode electrodes on the performance of microbial fuel cell. Biofuel Res. J. 2015, 2, 296–300. [Google Scholar] [CrossRef]










| Variable | Aeration Flow Rate | Anode pH | Cathode pH | Anode ORP | Cathode ORP | I Density |
|---|---|---|---|---|---|---|
| Aeration flow rate | - | - | - | - | - | - |
| Anode pH | 0.457 * | - | - | - | - | - |
| Cathode pH | 0.619 ** | 0.783 ** | - | - | - | - |
| Anode ORP | 0.111 | −0.362 * | −0.330 | - | - | - |
| Cathode ORP | 0.070 | 0.101 | 0.015 | 0.094 | - | - |
| I density | 0.317 | −0.533 ** | −0.236 | −0.095 | −0.099 | - |
| COD | - | 0.929 ** | 0.803 ** | −0.275 | - | −0.468 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatouq, A.; Babatunde, A.O. Concurrent Phosphorus Recovery and Energy Generation in Mediator-Less Dual Chamber Microbial Fuel Cells: Mechanisms and Influencing Factors. Int. J. Environ. Res. Public Health 2016, 13, 375. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13040375
Almatouq A, Babatunde AO. Concurrent Phosphorus Recovery and Energy Generation in Mediator-Less Dual Chamber Microbial Fuel Cells: Mechanisms and Influencing Factors. International Journal of Environmental Research and Public Health. 2016; 13(4):375. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13040375
Chicago/Turabian StyleAlmatouq, Abdullah, and Akintunde O. Babatunde. 2016. "Concurrent Phosphorus Recovery and Energy Generation in Mediator-Less Dual Chamber Microbial Fuel Cells: Mechanisms and Influencing Factors" International Journal of Environmental Research and Public Health 13, no. 4: 375. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13040375