Diet before and during Pregnancy and Offspring Health: The Importance of Animal Models and What Can Be Learned from Them
Abstract
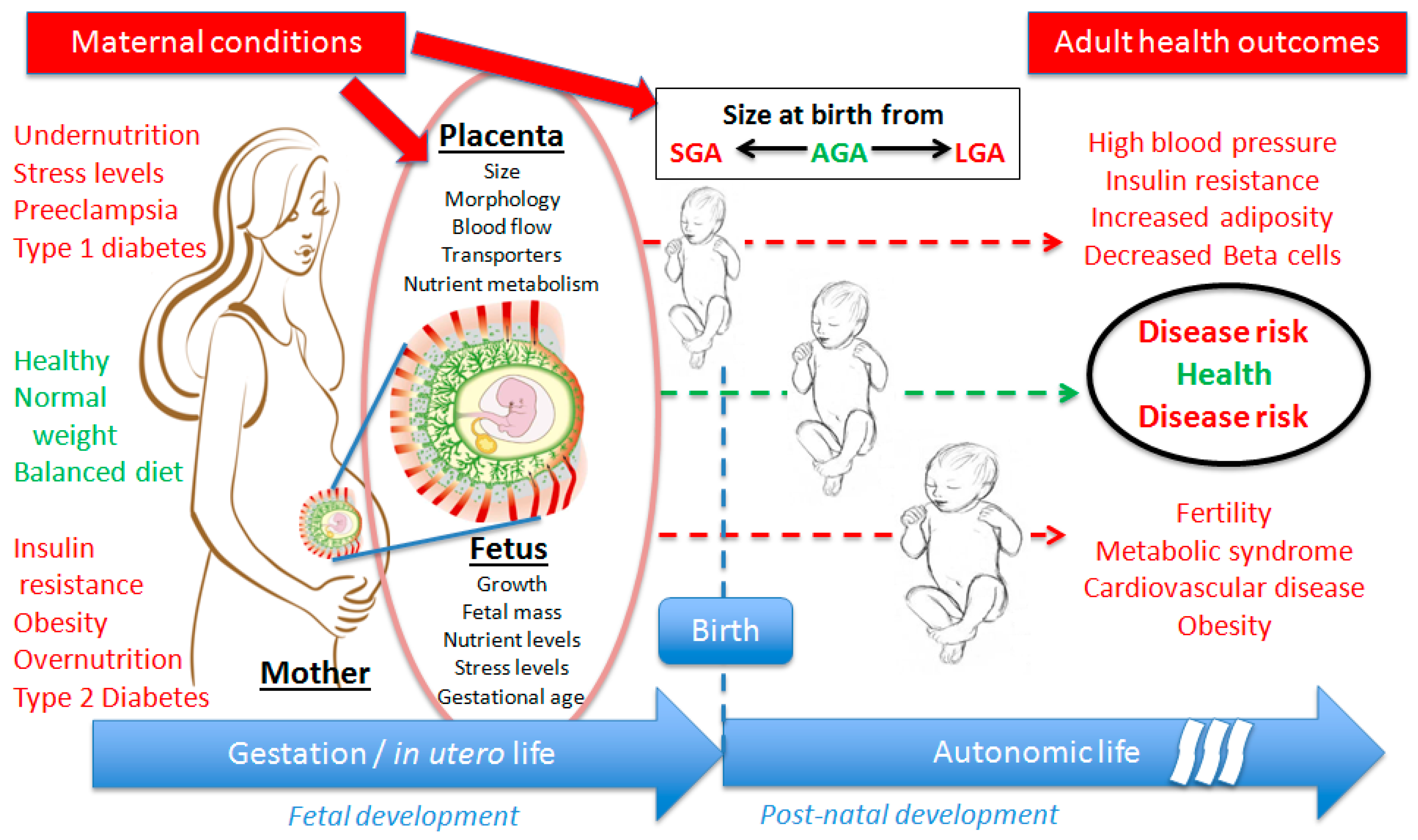
:1. Developmental Origins of Health and Diseases
2. Choice of Animal Models
3. Maternal Conditions Associated with DOHaD
3.1. Placental Insufficiency
3.2. Maternal Malnutrition
3.3. Maternal Overnutrition and/or Obesity
3.4. Maternal Diabetes
4. Interventional Experiments for Remediation of Long Term Effects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in england and wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Alfaradhi, M.Z.; Ozanne, S.E. Developmental programming in response to maternal overnutrition. Front. Genet. 2011, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin. Obstet. Gynecol. 2007, 50, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Manderson, J.G.; Mullan, B.; Patterson, C.C.; Hadden, D.R.; Traub, A.I.; McCance, D.R. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia 2002, 45, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007, 30, S169–S174. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Hauguel-De Mouzon, S. Is it time to revisit the pedersen hypothesis in the face of the obesity epidemic? Am. J. Obstet. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Sandboge, S.; Salonen, M.K.; Kajantie, E.; Osmond, C. Long-term consequences of maternal overweight in pregnancy on offspring later health: Findings from the helsinki birth cohort study. Ann. Med. 2014, 46, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Steegers, E.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The generation r study. Int. J. Obes. 2015, 39, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Gillman, M.W.; Mantzoros, C.S.; Oken, E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann. Epidemiol. 2014, 24. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, L.R.; Earl, S.; Cooper, C.; Harvey, N.C. Maternal diet, behavior and offspring skeletal health. Int. J. Environ. Res. Public Health 2010, 7, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Dibben, C.R.; Jones, P.B. Does maternal body mass index during pregnancy influence risk of schizophrenia in the adult offspring? Obes. Rev. Off. J. Int. Assoc. Study Obes. 2012, 13, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Dupont, C.; Chavatte-Palmer, P.; Gautier, B.; Levy, R.; Group, A.C. Are semen parameters related to birth weight? Fertil. Steril. 2015, 103, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Guillomot, M. Comparative implantation and placentation. Gynecol. Obstet. Investig. 2007, 64, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Moore, T. Maternal-fetal resource allocation: Co-operation and conflict. Placenta 2012, 33, e11–e15. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Panchenko, P.; Junien, C.; Gabory, A. Placental contribution to nutritional programming of health and diseases: Epigenetics and sexual dimorphism. J. Exp. Biol. 2015, 218, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Malassine, A.; Frendo, J.L.; Evain-Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 2003, 9, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Aigner, B.; Renner, S.; Kessler, B.; Klymiuk, N.; Kurome, M.; Wunsch, A.; Wolf, E. Transgenic pigs as models for translational biomedical research. J. Mol. Med. 2010, 88, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Whitelaw, C.B.; Sheets, T.P.; Lillico, S.G.; Telugu, B.P. Engineering large animal models of human disease. J. Pathol. 2016, 238, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Cost Action BM1308—Sharing Advances on Large Animal Models (SALAAM). Available online: http://www.salaam.genzentrum.lmu.de/ (accessed on 11 March 2016).
- Swanson, K.S.; Mazur, M.J.; Vashisht, K.; Rund, L.A.; Beever, J.E.; Counter, C.M.; Schook, L.B. Genomics and clinical medicine: Rationale for creating and effectively evaluating animal models. Exp. Biol. Med. 2004, 229, 866–875. [Google Scholar]
- Paigen, B. Genetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am. J. Clin. Nutr. 1995, 62, 458S–462S. [Google Scholar] [PubMed]
- Carter, A.M. Animal models of human placentation—A review. Placenta 2007, 28, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Laporte-Broux, B.; Tarrade, A.; Chavatte-Palmer, P. The use of ruminant models in biomedical perinatal research. Theriogenology 2012, 78, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, E.; Morel, O.; Tarrade, A.; Dahirel, M.; Bonneau, M.; Gayat, E.; Evain-Brion, D.; Chavatte-Palmer, P.; Tsatsaris, V. Quantification of utero-placental vascularization in a rabbit model of iugr with three-dimensional power doppler angiography. Placenta 2012, 33, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.W.; Wooding, F.B.; Fowden, A.L. Ovine feto-placental metabolism. J. Physiol. 2004, 554, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Neitzke, U.; Harder, T.; Schellong, K.; Melchior, K.; Ziska, T.; Rodekamp, E.; Dudenhausen, J.W.; Plagemann, A. Intrauterine growth restriction in a rodent model and developmental programming of the metabolic syndrome: A critical appraisal of the experimental evidence. Placenta 2008, 29, 246–254. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; MacLaughlin, S.M.; Muhlhausler, B.S.; Gentili, S.; Duffield, J.L.; Morrison, J.L. Developmental origins of adult health and disease: The role of periconceptional and foetal nutrition. Basic Clin. Pharmacol. Toxicol. 2008, 102, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Charnock-Jones, D.S.; Kaufmann, P.; Mayhew, T.M. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta 2004, 25, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Mayhew, T.M.; Charnock-Jones, D.S. Aspects of human fetoplacental vasculogenesis and angiogenesis. Ii. Changes during normal pregnancy. Placenta 2004, 25, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.; Milne, J.; Aitken, R.; Matsuzaki, M.; Reynolds, L.; Redmer, D.; Wallace, J. Placental growth, angiogenic gene expression, and vascular development in undernourished adolescent sheep. Biol. Reprod. 2007, 77, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Remacle, C.; Bieswal, F.; Bol, V.; Reusens, B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am. J. Clin. Nutr. 2011, 94, 1846S–1852S. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, S.E.; Hales, C.N. Lifespan: Catch-up growth and obesity in male mice. Nature 2004, 427, 411–412. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Field, M.E.; Anthony, R.V.; Engle, T.E.; Archibeque, S.L.; Keisler, D.H.; Han, H. Duration of maternal undernutrition differentially alters fetal growth and hormone concentrations. Domest. Animal Endocrinol. 2015, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morise, A.; Louveau, I.; Le Huerou-Luron, I. Growth and development of adipose tissue and gut and related endocrine status during early growth in the pig: Impact of low birth weight. Anim. Int. J. Anim. Biosci. 2008, 2, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Bispham, J.; Gopalakrishnan, G.S.; Dandrea, J.; Wilson, V.; Budge, H.; Keisler, D.H.; Broughton Pipkin, F.; Stephenson, T.; Symonds, M.E. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: Consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 2003, 144, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Mostyn, A.; Symonds, M.E. Early programming of adipose tissue function: A large-animal perspective. Proc. Nutr. Soc. 2009, 68, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Bee, G. Effect of early gestation feeding, birth weight, and gender of progeny on muscle fiber characteristics of pigs at slaughter. J. Anim. Sci. 2004, 82, 826–836. [Google Scholar] [PubMed]
- Rehfeldt, C.; Stabenow, B.; Pfuhl, R.; Block, J.; Nurnberg, G.; Otten, W.; Metges, C.C.; Kalbe, C. Effects of limited and excess protein intakes of pregnant gilts on carcass quality and cellular properties of skeletal muscle and subcutaneous adipose tissue in fattening pigs. J. Anim. Sci. 2012, 90, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.J.; Aitken, R.P.; Milne, J.S.; David, A.L.; Wallace, J.M. Fetoplacental biometry and umbilical artery doppler velocimetry in the overnourished adolescent model of fetal growth restriction. Am. J. Obstet. Gynecol. 2012, 207. [Google Scholar] [CrossRef] [PubMed]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Harrell, A.; Liu, X.; Gilchrist, J.M.; Ronis, M.J.; Badger, T.M. Maternal obesity at conception programs obesity in the offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R528–R538. [Google Scholar] [CrossRef] [PubMed]
- Picone, O.; Laigre, P.; Fortun-Lamothe, L.; Archilla, C.; Peynot, N.; Ponter, A.A.; Berthelot, V.; Cordier, A.G.; Duranthon, V.; Chavatte-Palmer, P. Hyperlipidic hypercholesterolemic diet in prepubertal rabbits affects gene expression in the embryo, restricts fetal growth and increases offspring susceptibility to obesity. Theriogenology 2011, 75, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Du, M.; Nathanielsz, P.W.; Ford, S.P. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 2010, 31, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, J.X.; Yan, X.; Zhu, M.J.; Long, N.M.; McCormick, R.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Maternal obesity enhances collagen accumulation and cross-linking in skeletal muscle of ovine offspring. PLoS ONE 2012, 7, e31691. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Grayton, H.; Poston, L.; Samuelsson, A.M.; Taylor, P.D.; Collier, D.A.; Rodriguez, A. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol. Psychiatr. 2012, 17, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P.; Zhang, L.; Zhu, M.; Miller, M.M.; Smith, D.T.; Hess, B.W.; Moss, G.E.; Nathanielsz, P.W.; Nijland, M.J. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: Prenatal consequences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R835–R843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, N.M.; Hein, S.M.; Ma, Y.; Nathanielsz, P.W.; Ford, S.P. Maternal obesity in ewes results in reduced fetal pancreatic beta-cell numbers in late gestation and decreased circulating insulin concentration at term. Domest. Anim. Endocrinol. 2011, 40, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhu, M.J.; Underwood, K.R.; Hess, B.W.; Ford, S.P.; Du, M. Amp-activated protein kinase and adipogenesis in sheep fetal skeletal muscle and 3t3-l1 cells. J. Anim. Sci. 2008, 86, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Soldado, I.; Herrera, E. Different diabetogenic response to moderate doses of streptozotocin in pregnant rats, and its long-term consequences in the offspring. Exp. Diabesity Res. 2003, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, N.D.; Corbo, L.; Stoddard, A.; Klein, A.H.; Tadokoro, N. Oxygen consumption and guanosine diphosphate binding by fetal brown adipose tissue in diabetic pregnancy. Metab. Clin. Exp. 1989, 38, 831–836. [Google Scholar] [CrossRef]
- Haucke, E.; Navarrete Santos, A.; Simm, A.; Henning, C.; Glomb, M.A.; Gurke, J.; Schindler, M.; Fischer, B.; Navarrete Santos, A. Accumulation of advanced glycation end products in the rabbit blastocyst under maternal diabetes. Reproduction 2014, 148, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Gauguier, D.; Bihoreau, M.T.; Ktorza, A.; Berthault, M.F.; Picon, L. Inheritance of diabetes mellitus as consequence of gestational hyperglycemia in rats. Diabetes 1990, 39, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Gauguier, D.; Bihoreau, M.T.; Picon, L.; Ktorza, A. Insulin secretion in adult rats after intrauterine exposure to mild hyperglycemia during late gestation. Diabetes 1991, 40, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.A.; McAuliffe, F.M.; Quinn, K.; Lonergan, P.; Evans, A.C. Transient high glycaemic intake in the last trimester of pregnancy increases offspring birthweight and postnatal growth rate in sheep: A randomised control trial. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Braun-Reichhart, C.; Streckel, E.; Renner, S. Genetically engineered pig models for diabetes research. Transgen. Res. 2014, 23, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.; Braun-Reichhart, C.; Blutke, A.; Herbach, N.; Emrich, D.; Streckel, E.; Wunsch, A.; Kessler, B.; Kurome, M.; Bahr, A.; et al. Permanent neonatal diabetes in ins(c94y) transgenic pigs. Diabetes 2013, 62, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- The 1,000 days. Available online: http://thousanddays.org/ (accessed on 11 March 2016).
- Nathanielsz, P.W.; Ford, S.P.; Long, N.M.; Vega, C.C.; Reyes-Castro, L.A.; Zambrano, E. Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr. Rev. 2013, 71, S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Heerwagen, M.J.; Stewart, M.S.; de la Houssaye, B.A.; Janssen, R.C.; Friedman, J.E. Transgenic increase in n-3/n-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS ONE 2013, 8, e67791. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, M.; Pfister, S.; Blutke, A.; Rozman, J.; Klingenspor, M.; Deutsch, M.J.; Rathkolb, B.; Fink, B.; Gimpfl, M.; Hrabe de Angelis, M.; et al. Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochim. Biophys. Acta 2014, 1842, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Kwong, W.Y.; Porter, R.; Ursell, E.; Fesenko, I.; Wilkins, A.; Miller, D.J.; Watkins, A.J.; Eckert, J.J. The embryo and its future. Biol. Reprod. 2004, 71, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Lucas, E.S.; Fleming, T.P. Impact of the periconceptional environment on the programming of adult disease. J. Dev. Orig. Health Dis. 2010, 1, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sasson, I.E.; Vitins, A.P.; Mainigi, M.A.; Moley, K.H.; Simmons, R.A. Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia 2015, 58, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Chillaron, J.C.; Isganaitis, E.; Charalambous, M.; Gesta, S.; Pentinat-Pelegrin, T.; Faucette, R.R.; Otis, J.P.; Chow, A.; Diaz, R.; Ferguson-Smith, A.; et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 2009, 58, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Pentinat, T.; Ribo, S.; Daviaud, C.; Bloks, V.W.; Cebria, J.; Villalmanzo, N.; Kalko, S.G.; Ramon-Krauel, M.; Diaz, R.; et al. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered lxra DNA methylation. Cell Metab. 2014, 19, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Owens, J.A.; Fullston, T.; Lane, M. Preconception diet or exercise intervention in obese fathers normalizes sperm microrna profile and metabolic syndrome in female offspring. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E805–E821. [Google Scholar] [CrossRef] [PubMed]


| Criteria | Mice | Rats | Rabbits | Pigs | Ruminants | Non-Human Primates | Comments |
|---|---|---|---|---|---|---|---|
| Cost | ++++ | +++ | + | + | - | The cost of mice being lower, more groups can be developed. | |
| Nutrition | +++ | +++ | + | ++++ | − | ++++ | Pigs have a digestive tract very similar to humans. Mice and rats can tolerate high fat diets. Pig and rabbit lipid metabolism are close to humans. |
| Pre-implantation development | +++ | ? | +++ | ? | ++ | ++ | The embryonic genome activation takes place at the 8–16 cell stages in all species, including humans, but for mice (2 cell stage). Mice are the best studied for preimplantation development. |
| Blastocyst stage | +++ | +++ | ++ | ++ | NA | Detailed knowledge of mice development is available. Rabbit blastocysts are used for fine analysis of gastrulation. They yield enough cells for individual embryo analysis. | |
| Placental physiology | +++ | +++ | ++ | ++++ | Primates, rabbits and rodents possess a hemochorial placentation (rabbit placenta being closest to humans). Ruminant and pig placenta are different. | ||
| Fetal development | ++ | ++ | ++ | +++ | ++++ | Polycotous species are less representative for human development compared to monocotous species. | |
| Management | ++++ | ++++ | ++++ | ++ | +++ | ++ | Small species are highly manageable, with short intergenerational intervals. In larger animals, ultrasound imaging of fetal growth is easily performed. |
| Lactation | ++ | +++ | ++++ | ++++ | +++ | +++ | In rabbits, suckling occurs only once a day, the study of milk intake and milk production easy. The rat “pup in the cup” model exists only in the rat. |
| Acceptability | ++++ | ++++ | ++++ | +++ | +++ | +/- | Mice and rats are the most acceptable. |
| Genomic tools | ++++ | +++ | ++ | +++ | +++ | +++ | Genomic and epigenetic tools and antibodies remain most available in mice, although possibilities increase in other species. |
| Transgenic models | ++++ | - | ++ | ++ | + | − | Transgenic mice models are largely available but transgenic models are also available in rabbits and pigs. |
| Programming outcomes that are identified through the measurements of physiological parameters in the offspring | |||||||
| Overweight | yes | yes | yes | yes | yes | yes | The ruminants have a different glucose metabolism from monogastric species and do not become diabetic. |
| Hypertension | yes | yes | yes | yes | yes | yes | |
| Diabetes | yes | yes | yes | yes | yes | ||
| Behavior | yes | yes | yes | yes | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavatte-Palmer, P.; Tarrade, A.; Rousseau-Ralliard, D. Diet before and during Pregnancy and Offspring Health: The Importance of Animal Models and What Can Be Learned from Them. Int. J. Environ. Res. Public Health 2016, 13, 586. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060586
Chavatte-Palmer P, Tarrade A, Rousseau-Ralliard D. Diet before and during Pregnancy and Offspring Health: The Importance of Animal Models and What Can Be Learned from Them. International Journal of Environmental Research and Public Health. 2016; 13(6):586. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060586
Chicago/Turabian StyleChavatte-Palmer, Pascale, Anne Tarrade, and Delphine Rousseau-Ralliard. 2016. "Diet before and during Pregnancy and Offspring Health: The Importance of Animal Models and What Can Be Learned from Them" International Journal of Environmental Research and Public Health 13, no. 6: 586. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060586






