The Combination of DGT Technique and Traditional Chemical Methods for Evaluation of Cadmium Bioavailability in Contaminated Soils with Organic Amendment
Abstract
:1. Introduction
2. Experimental
2.1. Soil Samples and Incubation
2.2. Greenhouse Pot Experiment
2.3. Analytical Methods of the Bioavailable Cd in Soils
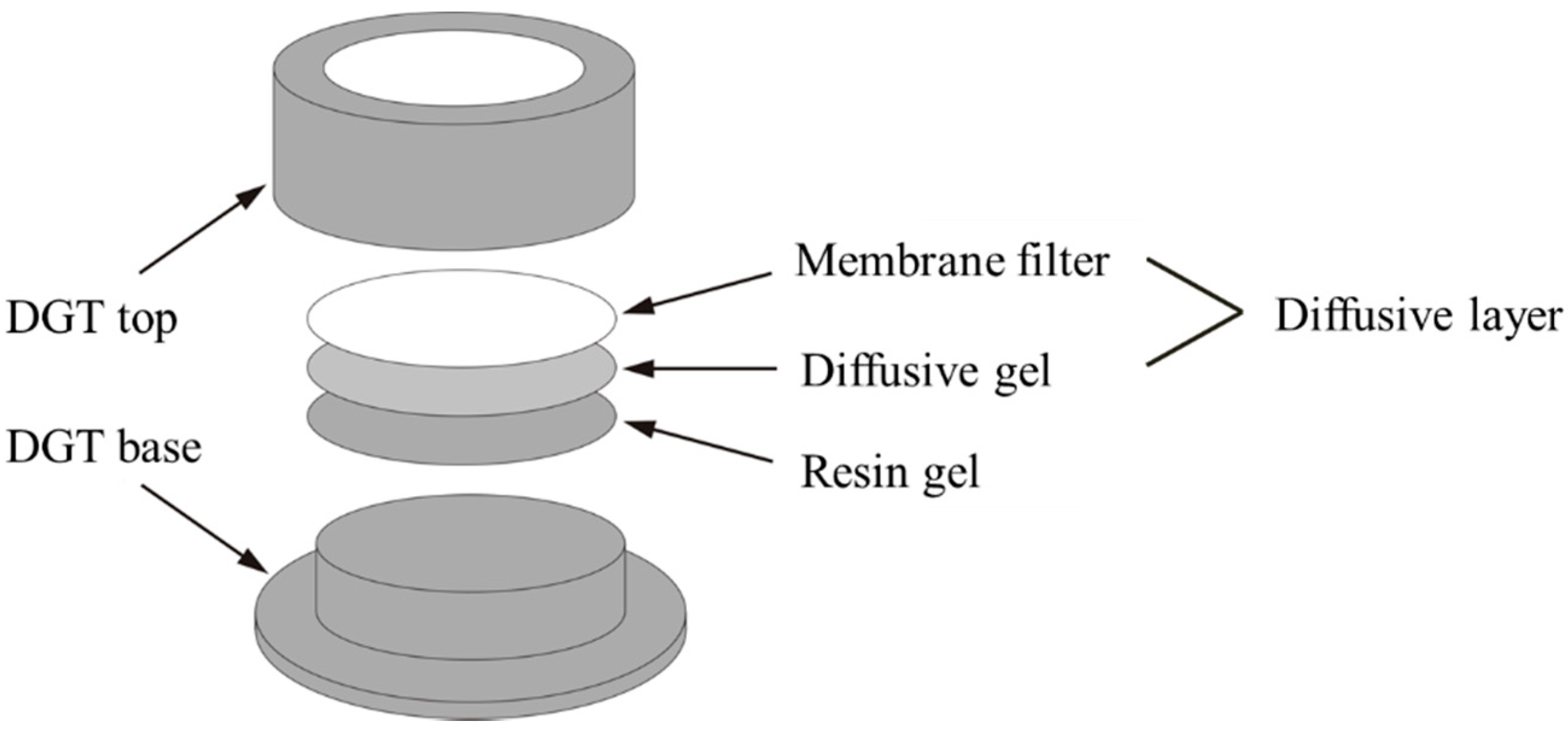
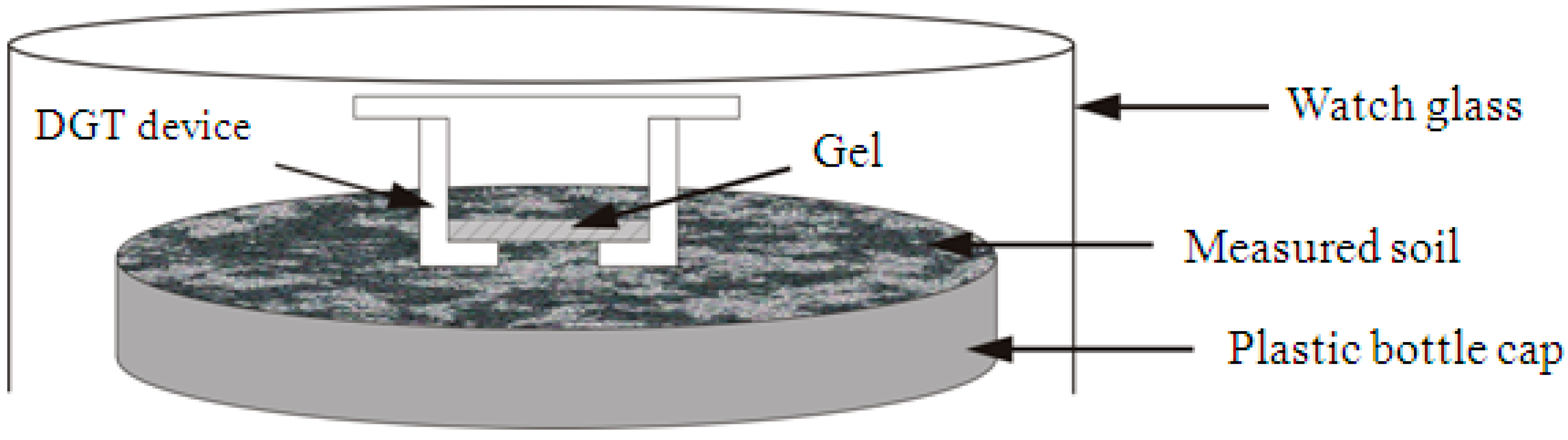
2.3.1. In Situ DGT Measurement
- 1.
- Subsample Moisture Adjustment
- 2.
- DGT Accumulation
- 3.
- Retrieval of the DGT Device
- 4.
- DGT Elution
- 5.
- Calculation of the DGT-Measured Concentrations
2.3.2. Ex Situ Measurement
Single Extraction Methods
Soil Solution Concentration
2.4. Data Analyses
3. Results and Discussion
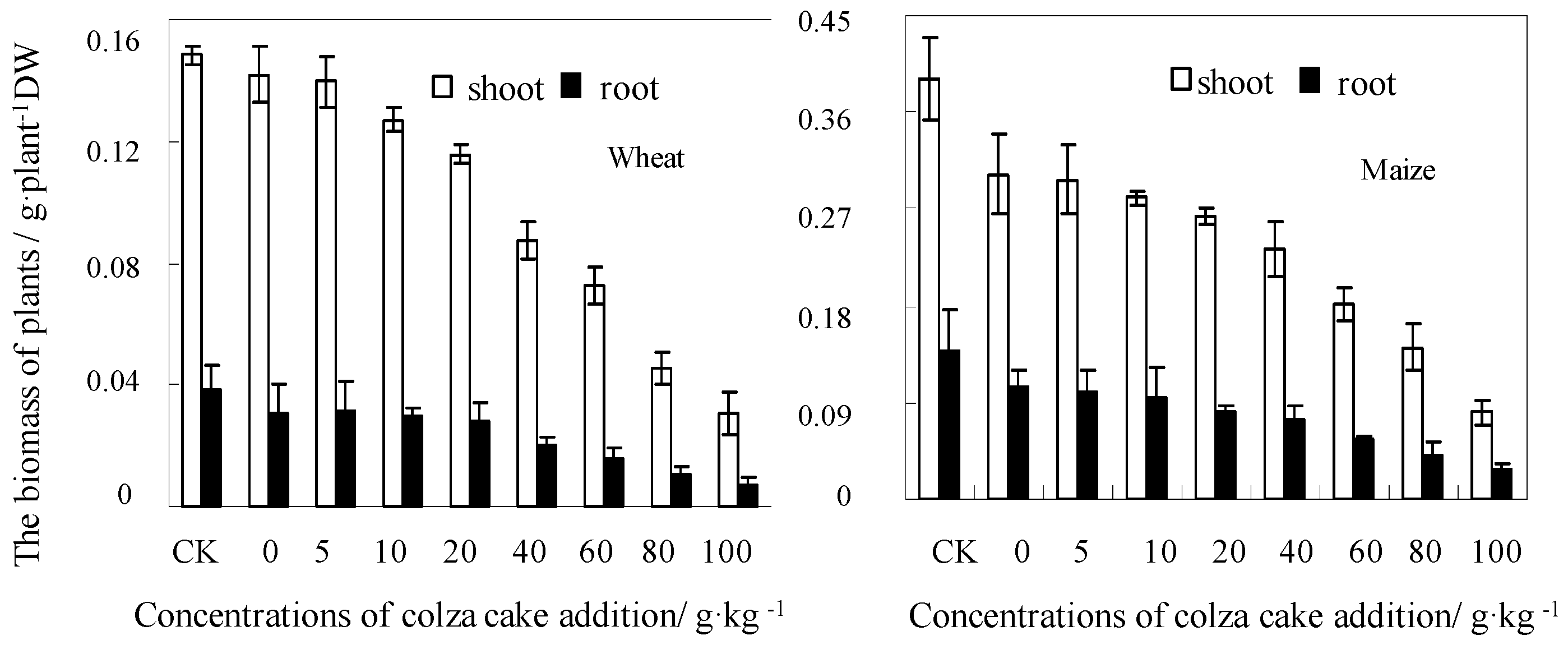
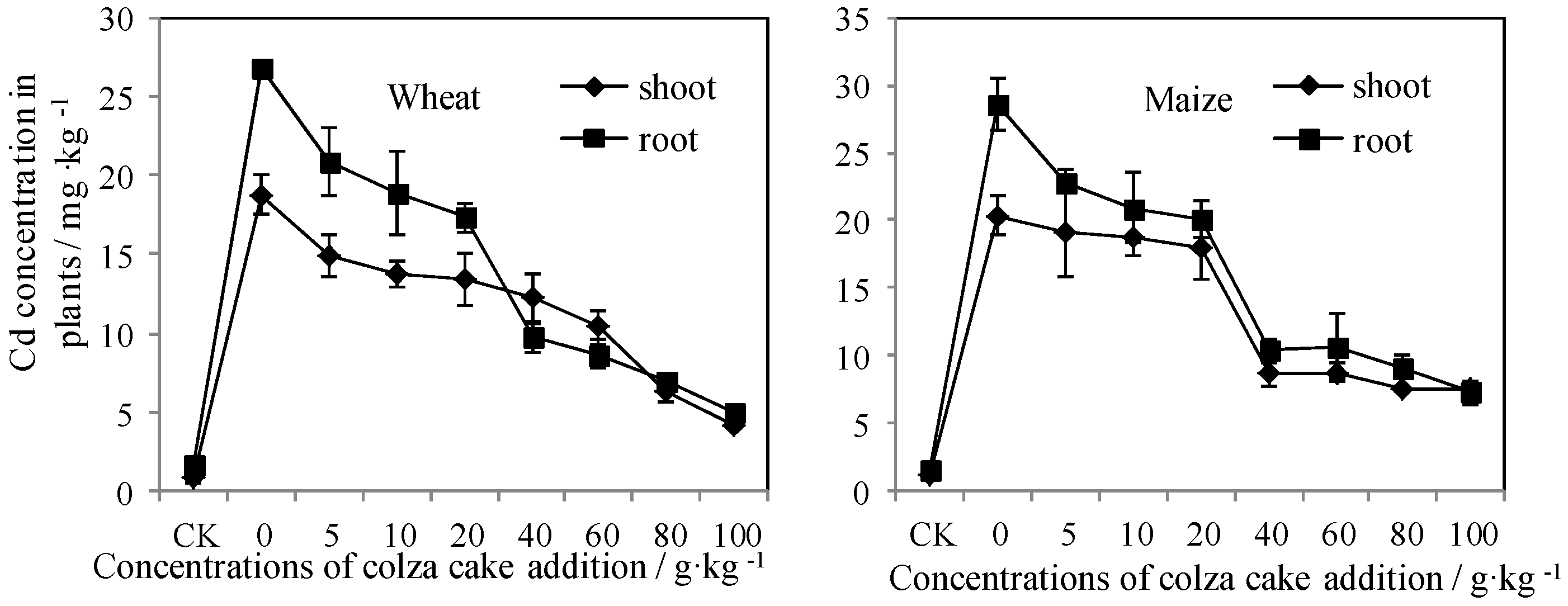
3.1. Plant Growth and Accumulation in Response to Colza Cake Exposure
3.2. Bioavailable Cd Reflected by a Dynamic Measurement
3.3. Bioavailable Cd Reflected by Static Measurements
3.3.1. Soil Solution Concentration of Cd
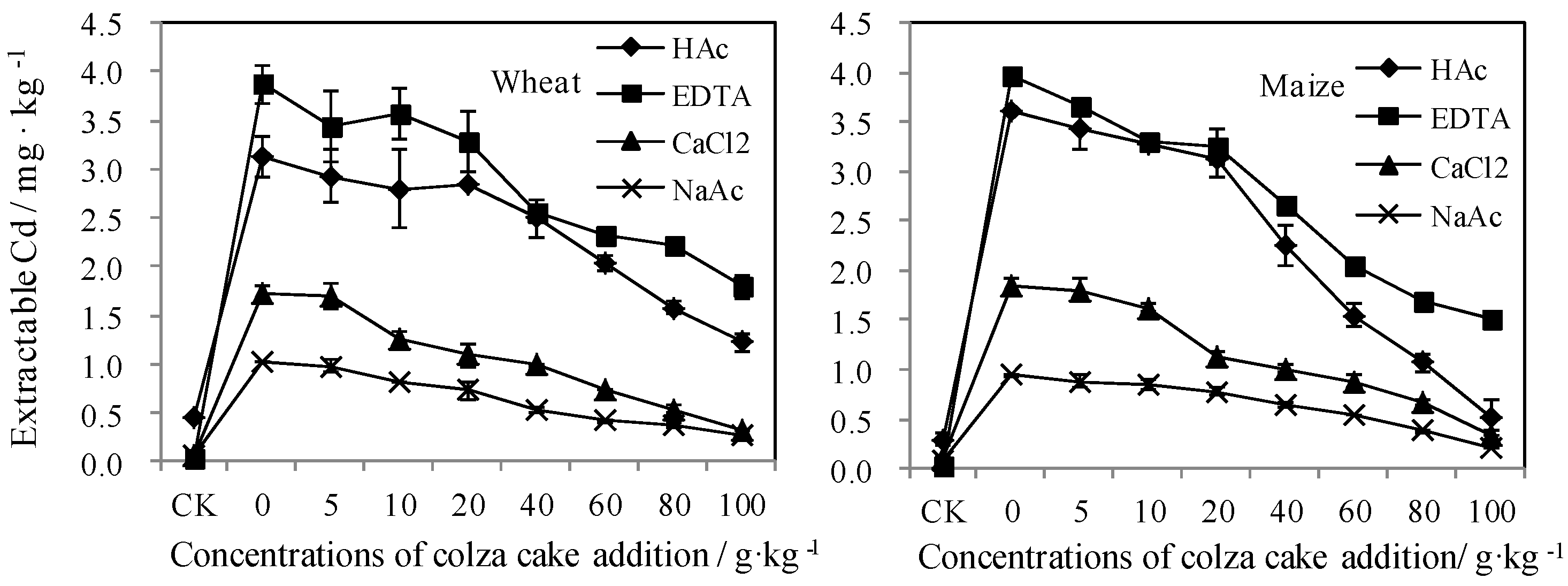
3.3.2. Bioavailable Cd Reflected by Traditional Extraction Measurements
3.4. The Inhibition to Cd Bioavailability Caused by Colza Cake
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on Cadmium Toxicity in Plants: A Review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Forsberg, L.S.; Kleja, D.B.; Greger, M. Effects of sewage sludge on solution chemistry and plant uptake of Cu in sulphide mine tailings at different weathering stages. Appl. Geochem. 2009, 24, 475–482. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, Q.; Wang, C.; Wang, P.F.; Ding, S.M. Evaluation of organic amendment on the effect of cadmium bioavailability in contaminated soils using the DGT technique and traditional methods. Environ. Sci. Pollut. Res. 2015, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lai, H.; Chen, Z. Effect of chemical amendments on the concentration of cadmium and lead in long-term contaminated soil. Chemosphere 2004, 57, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Zhao, F.J. Distinguishing diffusional and plant control of Cd and Ni uptake by hyper accumulator and nonhyper accumulator plants. Environ. Sci. Technol. 2010, 44, 6636–6641. [Google Scholar] [CrossRef] [PubMed]
- Hartley, W.; Edwards, R.; Lepp, W.N. Arsenic and heavy metal mobility in iron oxide-amended contaminated soil evaluated by short and long-term leaching tests. Environ. Pollut. 2004, 131, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Alloway, B.J. The role of dissolved organic carbon in the mobility of Cd, Ni and Zn in sewage sludge-amended soils. Environ. Pollut. 2002, 117, 515–521. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Sik, O.Y.; Charles, U.; Pittman, J. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Pietikainen, J.; Kiikkil, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. J. Hazard Mater. 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Venegas, A.; Rigol, A.; Vidal, M. Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere 2015, 119, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Dickinson, N.M. Carbon and trace element mobility in an urban soil amended with green waste compost. J. Soils Sediment. 2010, 10, 215–222. [Google Scholar] [CrossRef]
- Clemente, R.; Paredes, C.; Bernal, M.P. A field experiment investigating the effects of olive husk and cow manure on heavy metal availability in a contaminated calcareous soil from Murcia (Spain). Agric. Ecosyst. Environ. 2007, 118, 319–326. [Google Scholar] [CrossRef]
- Khokhotva, O.; Waara, S. The influence of dissolved organic carbon on sorption of heavy metals on urea-treated pine bark. J. Hazard Mater. 2010, 173, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, G.; De Imperial, R.M.; Torrijos, M. Effects of municipal solid waste compost and mineral fertilizer amendments on soil properties and heavy metals distribution in maize plants (Zea mays L.). Chemosphere 2011, 85, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarat, R.; Suebsima, R.; Putwattana, N. Effects of soil amendments on growth and metal uptake by Ocimumgratissimum grown in Cd/Zn-contaminated soil. Water Air Soil Pollut. 2011, 214, 383–392. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Nowack, B.; Koehler, S.; Schulin, R. Use of diffusive gradients in thin films (DGT) in undisturbed Field Soils. Environ. Sci. Technol. 2004, 38, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lombi, E.; Smolders, E. Kinetic of Zn release in soils and prediction of Zn concentration in plants using diffusive gradients in thin films. Environ. Sci. Technol. 2004, 38, 3608–3613. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, P.R.; Hayward, S.; Davison, W. In situ, high-resolution measurement of dissolved sulfide using diffusive gradients in thin films with computer imaging densitometry. Anal. Chem. 1999, 71, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Docekalova, H.; Divis, P. Application of diffusive gradient in thin films technique (DGT) to measurement of mercury in aquatic systems. Talanta 1999, 65, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Clarisse, O.; Hintelmann, H. Measurements of dissolved methylmercury in natural waters using diffusive gradients in thin film (DGT). Environ. Monit. 2006, 8, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Divis, P.; Szkandera, R.; Brulik, L. Application of New Resin Gels for Measuring Mercury by Diffusive Gradients ina Thin-films Technique. Anal. Sci. 2009, 25, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, X.R.; Luo, J. Evaluation of holistic approaches to predicting the concentrations of metals in field-cultivated rice. Environ. Sci. Technol. 2008, 42, 7649–7654. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z. Comparison of a rhizosphere-based method with Other One-Step Extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Menzies, N.W.; Donn, M.J.; Kopittke, P.M. Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ. Pollut. 2007, 145, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, A.; McLaren, R.G.; Reichman, S.M. Evaluation of soil metal bioavailablity estimates using two plant species (L. perenne and T. aestivum) grown in a range of agricultural soils treated with biosolids and metal salts. Environ. Pollut. 2011, 159, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Houba, V.J.G.; Lexmond, T.M.; Novozamsky, I. State of the art and future developments in soil analysis for bioavailability assessment. Sci. Total Environ. 1996, 178, 21–28. [Google Scholar] [CrossRef]
- DGT Research Limited Corporation. Available online: www.dgtresearch.com (accessed on 21 March 2016).
- Ure, A.M.; Quevauviller, P.H.; Muntau, H. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Wear, J.I.; Evans, C.E. Relationship of zinc uptake by corn and sorghum to soil zinc measured by three extractants. Soil Sci. Soc. Am. J. 1996, 32, 543–546. [Google Scholar] [CrossRef]
- Kaplan, O.; Yaman, M.; Kaya, G. Distribution of nickel in different phases of soil samples and plant parts taken from serpentine and copper mining area. Asian J. Chem. 2009, 21, 5757–5767. [Google Scholar]
- Novozamsky, I.; Lexmond, T.H.M.; Houba, V.J.G. A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. Int. J. Environ. Anal. Chem. 1993, 51, 47–58. [Google Scholar] [CrossRef]
- Kos, B.; Leštan, D. Induced phytoextraction/soil washing of lead using biodegradable chelate and permeablebarriers. Environ. Sci. Technol. 2003, 37, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Fornal, J.; Karamac, M. Effect of seed moisture on phenolic acids in rapeseed oil cake. Grasas Aceites 1995, 46, 354–356. [Google Scholar] [CrossRef]
- Tang, X.J.; Li, X.; Liu, X.M.; Muhammad, Z.H.; Xu, J.M.; Philip, C.B. Effects of inorganic and organic amendments on the uptake of lead and trace elements by Brassica Chinensis grown in an acidic red soil. Chemosphere 2015, 119, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- González-Núñez, R.; Alba, M.D.; Orta, M.M.; Rigol, A. Remediation of metal-contaminated soils with the addition of material—Part I: Characterization and viability studies for the selection of non-hazardous waste materials and silicates. Chemosphere 2011, 85, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Marijke, K.; Lucas, R.; Nathalie, R.; Willie, P. Comparison of the method of diffusive gels in thin films with conventional extraction techniques for evaluating zinc accumulation in plants and isopods. Environ. Pollut. 2005, 133, 103–116. [Google Scholar]
- Garmo, O.A.; Royset, O.; Steinnes, E. Performance study of diffusive gradients in thin films for 55 elements. Anal. Chem. 2003, 75, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B.; Nibarger, E.A.; Richards, B.K.; Steenhuis, T. Trace metal accumulation by red clover grown on sewage sludge-amended soils and correlation to Mehlich 3 and calcium chloride-extractable metals. Soil Sci. 2003, 168, 29–38. [Google Scholar] [CrossRef]
- Kamewada, K.; Nakayama, M. Cadmium uptake by garland chrysanthemum can be predicted from the cadmium in the soil solution, independent of soil type. Soil Sci. Plant Nutr. 2009, 55, 441–451. [Google Scholar]
- Paralelo, R.; Barral, M.T. Evaluation of the potential capacity as biosorbents of two MSW composts with different Cu, Pb and Zn concentration. Bioresour. Technol. 2012, 104, 810–813. [Google Scholar]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Sanka, M.; Dolezal, M. Prediction of Plant Contamination by Cadmium and Zinc Based on Soil Extraction Method and Contents in Seedlings. Int. J. Environ. Anal. Chem. 1992, 46, 87–96. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar]
- Payá-Pérez, A.; Sala, J.; Mousty, F. Comparison of ICP-AES and ICP-MS for the analysis of trace elements in soil extracts. Int. J. Environ. Anal. Chem. 1993, 51, 223–230. [Google Scholar]
- Bade, R.; Oh, S.; Shin, W.S. Assessment of metal bioavailability in smelter-contaminated soil before and after lime amendment. Ecotox. Environ. Saf. 2012, 80, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, H.S.; Cai, P.; Wei, L.; Huang, Q.Y. Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. J. Hazard Mater. 2009, 163, 563–567. [Google Scholar] [PubMed]
- Degryse, F.; Smolders, E.; Oliver, I. Relating soil solution Zn concentration to diffusive gradients in thin films measurements in contaminated soils. Environ. Sci. Technol. 2003, 37, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.P.; Davison, W.; Tych, W. DIFS-a modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils. Environ. Modell. Softw. 2000, 15, 55–66. [Google Scholar] [CrossRef]


| Extractants | Procedure | References |
|---|---|---|
| EDTA | 2.0 g of soil was extracted with 20 mL of 0.05 mol·L−1 EDT Aadjusted using an ammonia solution to pH = 7.0 and shaken for 2 h | Wear and Evans (1968) [32] |
| HOAc | 0.5 g of soil was extracted with 20 mL of 0.11 mol·L−1 HOAc and shaken for 16 h (overnight) | Houba et al. (1996) [29] |
| NaOAc | 4.0 g of soil was extracted with 20 mL of 1 mol·L−1 NaOAc and shaken for 2 h | Kaplan et al. (2009) [33] |
| CaCl2 | 2.0 g of soil was extracted with 20 mL of 0.01 mol·L−1 CaCl2 and shaken for 3 h | Novozamsky et al. (1993) [34] |
| Plant Species | Plant Tissues | DGT | Soil Solution | HAc | EDTA | NaAc | CaCl2 |
|---|---|---|---|---|---|---|---|
| wheat | shoot | 0.971 ** | 0.967 ** | 0.883 ** | 0.966 ** | 0.956 ** | 0.890 ** |
| root | 0.979 ** | 0.979 ** | 0.894 ** | 0.974 ** | 0.975 ** | 0.934 ** | |
| maize | shoot | 0.974 ** | 0.972 ** | 0.954 ** | 0.961 ** | 0.971 ** | 0.933 ** |
| root | 0.970 ** | 0.962 ** | 0.944 ** | 0.969 ** | 0.936 ** | 0.949 ** |
| Colza Cake Levels in Soil (g·kg−1) | Wheat | Maize |
|---|---|---|
| CK | 0.83 | 0.85 |
| 0 | 0.73 | 0.74 |
| 5.0 | 0.71 | 0.69 |
| 10.0 | 0.67 | 0.68 |
| 20.0 | 0.63 | 0.65 |
| 40.0 | 0.51 | 0.52 |
| 60.0 | 0.37 | 0.29 |
| 80.0 | 0.31 | 0.21 |
| 100.0 | 0.27 | 0.16 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Sun, Q.; Wang, C.; Wang, P.-F.; Miao, L.-Z.; Ding, S.-M. The Combination of DGT Technique and Traditional Chemical Methods for Evaluation of Cadmium Bioavailability in Contaminated Soils with Organic Amendment. Int. J. Environ. Res. Public Health 2016, 13, 595. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060595
Yao Y, Sun Q, Wang C, Wang P-F, Miao L-Z, Ding S-M. The Combination of DGT Technique and Traditional Chemical Methods for Evaluation of Cadmium Bioavailability in Contaminated Soils with Organic Amendment. International Journal of Environmental Research and Public Health. 2016; 13(6):595. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060595
Chicago/Turabian StyleYao, Yu, Qin Sun, Chao Wang, Pei-Fang Wang, Ling-Zhan Miao, and Shi-Ming Ding. 2016. "The Combination of DGT Technique and Traditional Chemical Methods for Evaluation of Cadmium Bioavailability in Contaminated Soils with Organic Amendment" International Journal of Environmental Research and Public Health 13, no. 6: 595. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060595






