1. Introduction
Prevalence of overweight and obesity, which are established risk factors for non-communicable diseases such as cardiovascular diseases, diabetes, and certain cancers, has dramatically increased around the world since 1980. In 2014, 39% and 13% of adults (above 20 years old) were overweight and obese worldwide, respectively [
1]. Fortunately, evidence shows that obesity can be prevented by changing potential causes such as dietary behavior and physical activity [
2].
The fundamental cause of obesity is an energy imbalance between energy intake (EI) and energy expenditure (EE). Weight gain indicates that the balance has tipped towards increased EI or reduced EE or a combination of both [
3]. In humans, when EI exceeds EE by 11 kcal per day, a one pound weight gain will occur over the course of a year [
4]. It has been indicated that ingestive behaviors such as choice of foods, time to consume a meal, as well as meal size and function of gastrointestinal system influence EI [
5]. For example, EE can drop when there is an increase of sedentary behavior and elevated use of vehicles, which are associated with a decrease of physical activity. Recently, there have been a number of studies investigating the effect of changing dietary behaviors on EI, which is one of goals of the present study.
One of the modifiable dietary behaviors is eating frequency (EF). Commonly, an eating occasion is defined as any instance in which participants reported consumption of solid meals and snacks, with a minimum gap of 15 min between two eating episodes [
6]. However, definition of eating occasion can be different, depending on different research. Currently, the relationship between EF and EI is controversial. Animal experiments found that increased EF was associated with higher EI in mice [
7], which was in line with observation in human [
8]. However, Speechly
et al. reported that increased EF improved appetite control and subsequently reduced EI in obese males [
9]. Interestingly, it has been suggested that increased EF had minimal effect on appetite and EI but reduced EF showed negative impact on appetite and EI based on controlled feeding studies in humans [
10].
Despite conclusion about the association between EF and EI is far from consistent, it is plausible that EF may influence at least one side of energy balance and thus affects body weight. Previous evidence from animal studies suggested that the effect of EF on energy balance and body composition was not obvious in rats during caloric restriction [
11]. This provided implications for the research on the relationship between EF and body weight without food restriction. Verbaeys
et al. found that without caloric restriction on a daily basis, rats fed
ad libitum gained weight faster than three-time schedule-fed rats [
12]. For humans, the mainstream opinion is that increased EF is associated with a healthier body weight status in both children and adults [
13,
14,
15]. Since Fabry
et al. pointed out this inverse relationship in the 1960s [
16], numerous cross-sectional studies had been carried out and similar results had been obtained. Recently, Kaisari
et al. have shown that increased EF was associated with lower obesity risk in children and adolescents, especially in boys [
17]. Similarly, Schoenfeld
et al. suggested a potential benefit of higher EF for body weight status during weight loss, although the positive findings were produced by a single study [
18]. However, several studies demonstrated null [
19,
20] or positive relationship [
21,
22,
23] between EF and body weight. Duval
et al. suggested that after correcting the effect of physical activity EE, the association between EF and obesity was no longer significant [
24]. The present study aims to evaluate whether EF is associated with obesity risk and EI in adults without caloric restriction by conducting a meta-analysis of published original observational studies.
2. Methods
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [
25].
2.1. Search Strategy
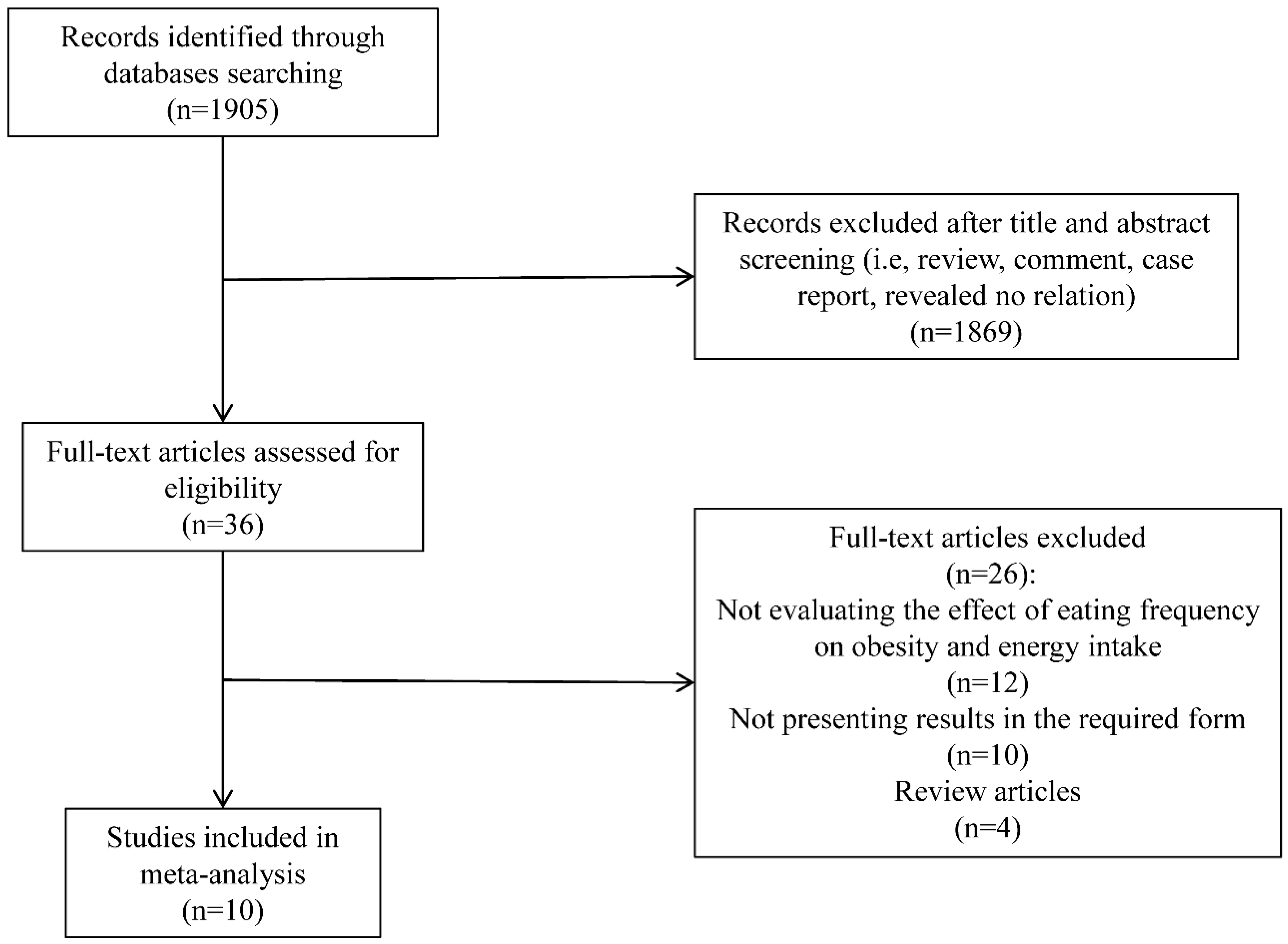
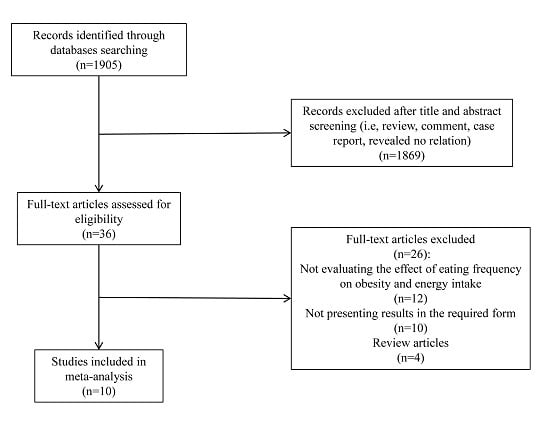
Original research and observational studies (including those identified via review articles) published before July 2015, examining the association between EF and obesity in adults who were not during weight loss, were selected through English-language literature searches in the PubMed, Elsevier Science Direct, Nature, Science Online and Embase databases. Combinations of at least two of the following key words were used as search terms: meal frequency, feeding frequency, EF, eating patterns, eating behaviors, body composition, weight, body mass index (BMI), obesity and EI. In addition, the reference lists of the eligible retrieved articles were used to identify relevant articles that were not extracted through the searching procedure. Abstracts from conferences, reviews, and unpublished dissertations or theses were excluded from analysis. To reduce the potential for selection bias, each study was independently evaluated by 2 of the investigators (Yue-Qiao Wang and Yi-Wen Zhang), and a mutual decision was made jointly as to whether or not it met the basic inclusion criteria. The disagreements were solved by the opinion of the third author (Fei Zhang), and consensus was reached by discussion.
2.2. Inclusion Criteria
Studies were included if they met the following criteria: (1) original research or observational studies published in an English-language refereed journal; (2) all of the selected studies were conducted in adults; (3) participants or subjects were not during weight loss and diet control; (4) participants or subjects did not have psychological and eating disorders; (5) no experience of bariatric surgery; (6) non-pregnancy; (7) the independent variable was EF; (8) the dependent variable was risk of obesity or EI; (9) results were provided in a form that could be used for the present analysis (point estimates of odd ratios (OR) with 95% confidence intervals (CI) or the differences in the mean values of the EI among EF categories and standard errors (SEs) available).
2.3. Data Extraction
The following information was extracted from each included study: first author’s last name; year of publication; country of origin; sample size; age and gender of participants, and the number of daily meals/eating episodes; effect size measurements; 95% CIs; SEs or SDs; the evaluation of EF and EI; the definition of a meal/eating episode and variables that entered into the multivariable model as potential confounding factors. Information and data was extracted by one author (Yue-Qiao Wang) and checked by another (Yun-Quan Zhang). Quality of studies was not assessed because the total number of studies was limited.
2.4. Quality Assessment
Individual study quality was assessed using a score modified from the Agency for Healthcare Research and Quality (AHRQ) Cross-Sectional Study Quality Assessment [
26]. For each study, the appropriateness in dealing with eleven items was checked as follows: (1) define the source of information; (2) list inclusion and exclusion criteria for exposed and unexposed subjects or refer to previous publications; (3) indicate time period used for identifying patients; (4) indicate whether or not subjects were consecutive if not population-based; (5) indicate if evaluators of subjective components of study were masked to other aspects of the status of participants; (6) describe any assessments undertaken for quality assurance purposes; (7) explain any patient exclusions from analysis; (8) describe how confounding was assessed and/or controlled; (9) if applicable, explain how missing data were handled in the analysis; (10) summarize patient response rates and completeness of data collection; (11) clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained. For item 5, a score of 1 was assigned when answer was no, and for other items, a score of 1 was assigned when answer was yes.
2.5. Statistical Analysis
For studies investigating the relationship between EF and obesity risk, the effect size was presented as ORs and their corresponding 95% CIs. The lowest category was considered as reference group. If the original study used the highest category as reference, reciprocal of original ORs was calculated for analysis. Single comparison between one EF category
versus the reference constituted the units of the meta-analysis. When more than one comparisons of different EF categories were included in the same study, an overall estimate for the study was calculated from ORs using the fixed-effect model [
27]. For example, if the EF categories included ≤3; 4–5 and ≥6 and EF ≤ 3 was considered as reference group, we used fixed-effect model to combine ORs of other two groups as overall estimate.
For studies investigating the relationship between EF and EI, the effect size was shown as the differences of EI among categories and their corresponding SEs. If the study reported EI in kilojoule or megajoule, we unified the unit as Kcal. In each study, we calculated the differences of EI among categories. For example, if the EF categories included ≤3; 4–5 and ≥6, we calculated the differences of EI between each two groups as well as their corresponding SEs, and computed the overall estimate from the difference of EI using the fixed-effect model. If SEs or SDs were not reported, SEs were computed from 95% CI following a standard methodology [
27].
If a paper reported the results of different multivariate models, the most stringently controlled estimate were extracted. Statistical heterogeneity that was attributed to studies rather than to chance was assessed by visual inspection of forest plots as well as using Cochran’s
Q and
I2, and evaluated by performing the χ
2 test (assessing the
p value) [
28]. If the
p value was less than 0.10 and
I2 exceeded 50%, indicating the presence of heterogeneity, a random-effects model (the DerSimonian and Laird method) was used [
29]; otherwise, the fixed-effects model (the Mantel–Haenszel method) was used [
30]. The presence of publication bias was carried out by visual inspection of asymmetric plots, and tested with the Egger linear regression method of asymmetry [
31,
32,
33]. All statistical calculations were performed in R software (R Foundation for Statistical Computing, Vienna, Austria).
4. Discussion
To the best of our knowledge, this is the first meta-analysis to assess the effect of EF on obesity risk and EI in adults without caloric restriction. We found a significant and inverse relationship between EF and obesity risk. Adults with high EF had 17% lower probabilities of getting overweight and obese. Our analysis also indicated a significant and positive association between EF and EI in adults.
Several theories have been proposed to explain the negative relationship between EF and obesity risk in adults. For instance, it has been reported that increased EF improves dietary quality and is associated with lower dietary energy density [
6] and therefore lower BMI [
43,
44,
45]. Dietary quality refers to the nutrition component of a diet and is measured by evaluating dietary patterns based on national dietary guidelines [
46,
47]. According to the Healthy Eating Index (HEI) that is developed to evaluate overall diet quality, high dietary quality requires 30% or less EI from fat, less than 10% EI from saturated fat and low protein intake [
48]. High EF is usually associated with high EI from carbohydrates but low EI from protein and fat due to the increased consumption of healthy snacks like fruits [
34,
35,
49]. Additionally, high EF may significantly increase the thermic effect of food, which is an important component of EE. Time of eating might be another cause of the negative association between EF and obesity. High EF is usually associated with more eating occasions occurring early in a day, which is more likely to promote EE. Therefore, higher EF may lead to lower obesity risk.
Increased EF may influence hormone secretion and nutrient metabolism, which play important roles in obesity. It has been suggested that increased EF is associated with a reduction in total insulin secretion and better blood glucose control [
50]. Jenkins
et al. found that during a high EF diet, the mean serum insulin level was decreased by approximately 28% [
51]. Karatzi
et al. recruited 164 healthy subjects, measured their plasma levels of insulin and glucose, and found that EF was inversely correlated with insulin concentration and postprandial glucose [
38]. Additionally, increased EF may lead to lower serum lipid concentrations [
34,
51], lower concentrations of total cholesterol and low-density-lipoprotein cholesterol [
6,
41,
52]. These evidence suggest that high EF has a positive effect on nutrient metabolism, which directly contribute to obesity risk.
Several epidemiological studies have suggested that the negative relationship between EF and obesity risk is attributed to reporting bias [
20,
53], considering that obese and elder individuals may under-report food consumption [
14,
54]. However, the same association was observed from researches excluding under-reporters [
14,
34].
It is worth noting that Drummond
et al. reported this inverse relationship between EF and obesity in males, but not in females [
14]. Similar results were obtained from a meta-analysis of the relationship between EF and obesity in children [
17], indicating that gender difference should be considered as a cofounding factor in the analysis. However, due to the limited studies in this area, we were unable to analyze the differential effects of EF on obesity risk in males and females.
Our results support a positive relationship between EF and EI. One plausible explanation for the positive association between EF and EI is that higher EF may increase the probability of excessive energy consumption [
39]. However, it has been reported that high EF promotes appetite control, and this may result in reduced EI [
9,
55,
56]. Additionally, a recent study has shown that high EF has little influence on appetite control based on an 8-week intervention trial [
57]. We speculated that another factor, portion size, might contribute to the reported differential relationships between EF and EI [
58]. The portion size of foods, which is commercially available at supermarkets, family-type restaurants and fast food establishments, has been identified as an important factor to affect EI [
59,
60]. However, despite the effects of portion size should not be neglected, mechanistic evidence suggested that compensatory dietary response to increased EF was weaker than to larger portion size, making high EF a more sensitive factor for EI [
61].
It is of interest to find that increased EF is associated with greater EI but lower obesity risk. Previous studies have reported that individuals with higher EF are likely to live a more active lifestyle, leading to higher EI and EE [
34,
38]. In addition, Duval
et al. reported that physical activity EE was positively correlated with EF by using more objective measures [
24]. This finding may partially explain the different effects of EF on obesity risk and EI. Regarding this discrepancy, an alternative explanation may be that errors commonly attributed to dietary assessment might have occurred during the assessment of these two outcomes. Additionally, difference in dietary quality may also play a role. For example, subjects involved in the studies with higher EF and EI may be eating a diet with relative low caloric content. Nevertheless, the underlying mechanism merits further investigation.
This meta-analysis has several potential limitations. First, only observational studies were included in the analysis and therefore casual inference was limited. For instance, increased EF could be both a cause and consequence of obesity, since obese individuals might increase EF to control body weight. Second, we could only identify cross-sectional studies based on our search strategy because very few studies investigated the associations between EF and obesity risk and/or EI in randomized clinical trials (RCTs). We realized that the evidence level of cross-sectional studies was not as convinced as RCTs and cohort studies; however, considering the current research in EF, it is difficult to perform a meta-analysis on RCTs or cohort studies due to the lack of those studies at the time of our analysis. Third, although we performed subgroup analyses, we were unable to assess the exact effect of every exposure on the observed relationship due to limited selected studies. Third, inconsistent definition for EF should be taken into account. Also, the validity and reliability of food frequency questionnaire and dietary recall to evaluate EF and EI need to be considered. However, this is a common limitation in reviews and meta-analysis evaluating dietary behaviors [
62], since there is no general agreement on the appropriate definition and assessment strategy [
63]. Finally, misinformation of EI and physical activity was not excluded in several selected studies.