Areas with High Hazard Potential for Autochthonous Transmission of Aedes albopictus-Associated Arboviruses in Germany
Abstract
:1. Introduction
2. Materials and Methods
3. Results
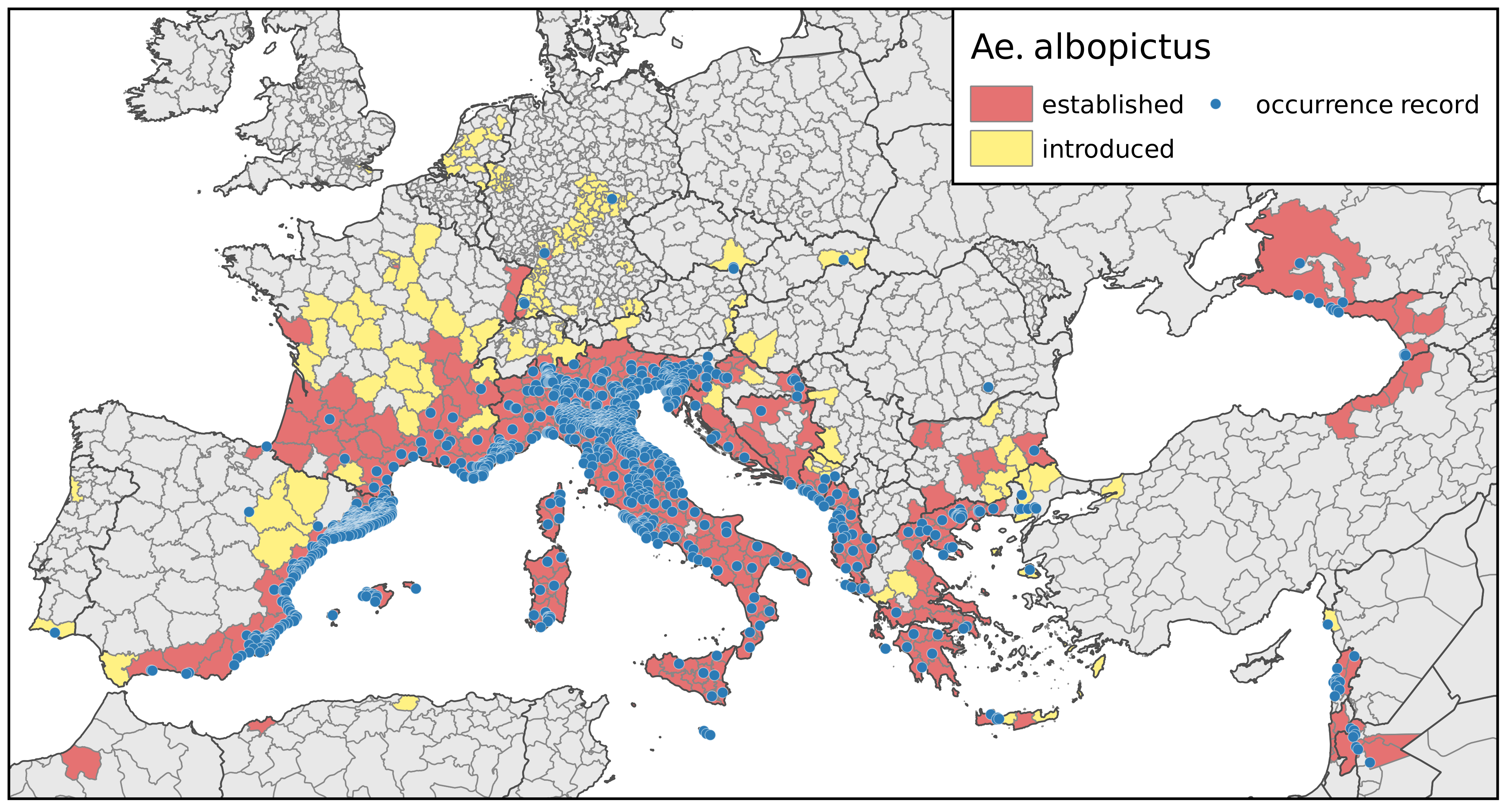
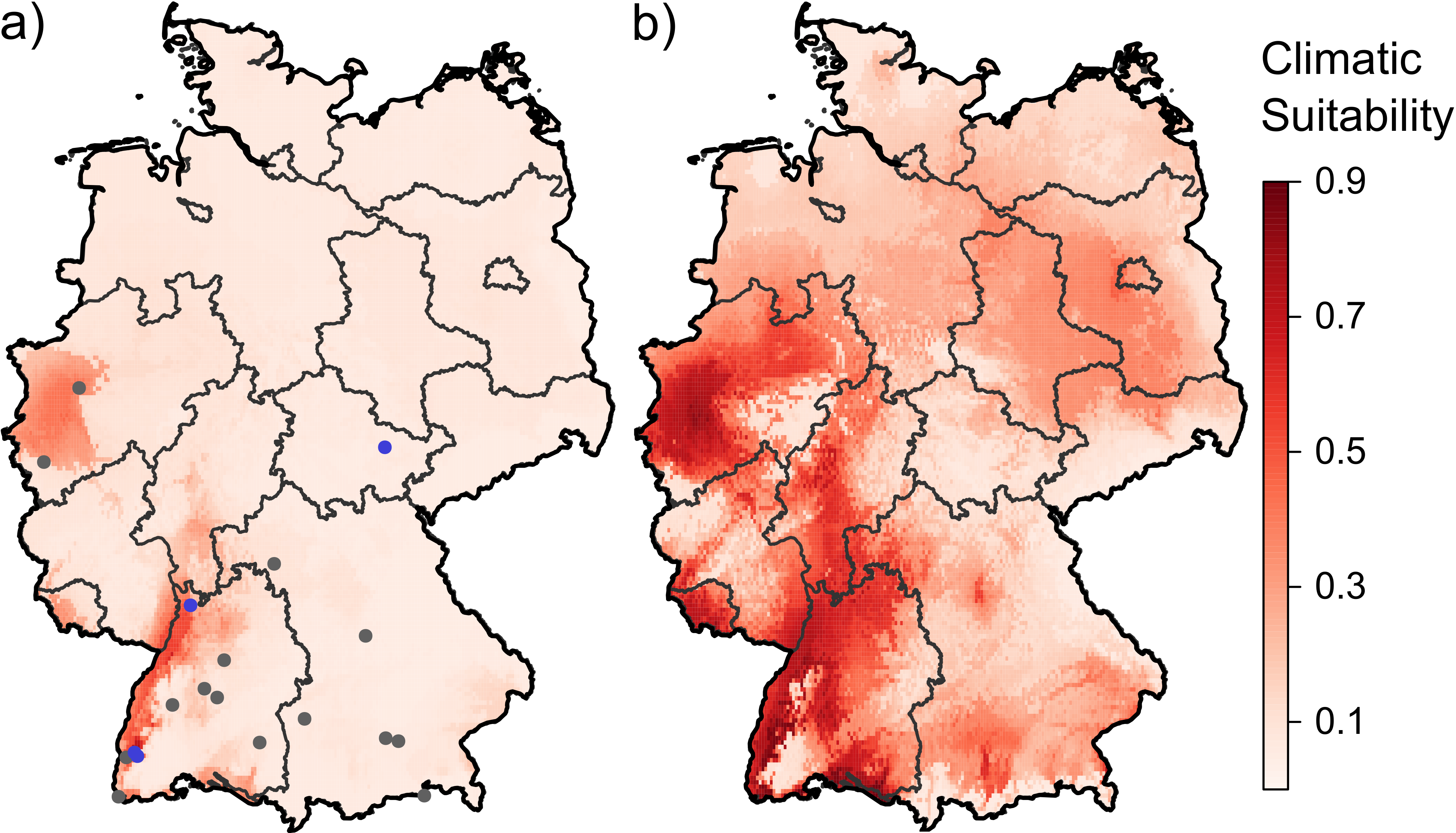
3.1. Current and Future Climatically Suitable Areas for the Establishment of Aedes albopictus
3.2. German Counties and Population Showing a Hazard Potential for Autochthonous Transmission of Dengue and Chikungunya Viruses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, N.B.; Suk, J.E.; Fischer, D.; Thomas, S.M.; Beierkuhnlein, C.; Semenza, J.C. Modelling the effects of global climate change on Chikungunya transmission in the 21st century. Sci. Rep. 2017, 7, 38813. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahola, T.; Courderc, T.; Ng, L.F.P.; Hallengard, D.; Powers, A.; Lecuit, M.; Esteban, M.; Merits, A.; Roques, P.; Liljestrom, P. Therapeutics and vaccines against chikungunya virus. Vector Borne Zoonotic Dis. 2015, 15, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Bortel, W.V. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.M.; Obermayr, U.; Fischer, D.; Kreyling, J.; Beierkuhnlein, C. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasit. Vectors 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microb. Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, A.; Jansen, S.; Luhken, R.; Leggewie, M.; Badusche, M.; Pluskota, B.; Becker, N.; Vapalahti, O.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of Zika virus by mosquitoes from central Europe. Eurosurveill 2017, 22, 30437. [Google Scholar] [CrossRef] [PubMed]
- Angelini, R.; Finarelli, A.C.; Angelini, P.; Po, C.; Petropulacos, K.; Macini, P.; Fiorentini, C.; Fortuna, C.; Venturi, G.; Romi, R.; et al. An outbreak of chikungunya fever in the province of Ravenna, Italy. Eurosurveill 2007, 12, E070906. [Google Scholar] [CrossRef]
- Grandadam, M.; Caro, V.; Plumet, S.; Thiberge, J.M.; Souares, Y.; Failloux, A.B.; Tolou, H.J.; Budelot, M.; Cosserat, D.; Leparc-Goffart, I.; et al. Chikungunya virus, southeastern France. Emerg. Infect. Dis. 2011, 17, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Delisle, E.; Rousseau, C.; Broche, B.; Leparc-Goffart, I.; L’Ambert, G.; Cochet, A.; Prat, C.; Foulongne, V.; Ferre, J.B.; Catelinois, O.; et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Eurosurveill 2015, 20, 21108. [Google Scholar] [CrossRef] [Green Version]
- Calba, C.; Guerbois-Galla, M.; Franke, F.; Jeannin, C.; Auzet-Caillaud, M.; Grard, G.; Pigaglio, L.; Decoppet, A.; Weicherding, J.; Savaill, M.C.; et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Eurosurveil 2017, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. Clusters of Autochthonous Chikungunya Cases in Italy, First Update—9 October 2017. Available online: https://ecdc.europa.eu/en/publications-data/rapid-risk-assessment-clusters-autochthonous-chikungunya-cases-italy-first-update (accessed on 18 April 2018).
- La Ruche, G.; Souares, Y.; Armengaud, A.; Peloux-Petiot, F.; Delaunay, P.; Despres, P.; Lenglet, A.; Jourdain, F.; Leparc-Goffart, I.; Charlet, F.; et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Eurosurveill 2010, 15, 19676. [Google Scholar] [CrossRef]
- Gjenero-Margan, I.; Aleraj, B.; Krajcar, D.; Lesnikar, V.; Klobucar, A.; Pem-Novosel, I.; Kurecic-Filipovic, S.; Komparak, S.; Martic, R.; Duricic, S.; et al. Autochthonous dengue fever in Croatia, August-September 2010. Eurosurveill 2011, 16, 19805. [Google Scholar] [CrossRef]
- Marchand, E.; Prat, C.; Jeannin, C.; Lafont, E.; Bergmann, T.; Flusin, O.; Rizzi, J.; Roux, N.; Busso, V.; Deniau, J.; et al. Autochthonous case of dengue in France, October 2013. Eurosurveil 2013, 18, 206. [Google Scholar] [CrossRef]
- Succo, T.; Leparc-Goffart, I.; Ferre, J.; Roiz, D.; Broche, B.; Maquart, M.; Noel, H.; Catelinois, O.; Entezam, F.; Caire, D.; et al. Autochthonous dengue outbreak in Nimes, South of France, July to September 2015. Eurosurveill 2016, 21, 3024. [Google Scholar] [CrossRef] [PubMed]
- Pluskota, B.; Storch, V.; Braunbeck, M.; Becker, N. First record of Stegomyia albopicta (Skuse) (Diptera: Culicidae) in Germany. Eur. Mosq. Bull. 2008, 26, 1–5. [Google Scholar]
- Sebesta, O.; Rudolf, I.; Betasova, L.; Pesko, J.; Hubalek, Z. An invasive mosquito species Aedes albopictus found in the Czech Republic, 2012. Eurosurveill 2012, 17, 20301. [Google Scholar] [CrossRef]
- Seidel, B.; Nowotny, N.; Indra, A.; Allerberger, F. Emergence of the asian tiger mosquito, Aedes (Stegomyia) albopictus (Diptera: Culicidae) in two geographically separated Austrian provinces, May and September 2012. Beitr. Entomofaunist. 2015, 16, 83–88. [Google Scholar]
- Werner, D.; Kampen, H. Aedes albopictus breeding in southern Germany, 2014. Parasitol. Res. 2015, 114, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Kronefeld, M.; Schaffner, F.; Kampen, H. Two invasive mosquito species, Aedes albopictus and Aedes japonicus japonicus, trapped in south-west Germany, July to August 2011. Eurosurveill 2012, 17, 20067. [Google Scholar] [CrossRef] [Green Version]
- Becker, N.; Geier, M.; Balczun, C.; Bradersen, U.; Huber, K.; Kiel, E.; Kruger, A.; Luhken, R.; Orendt, C.; Plenge-Bonig, A.; et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol. Res. 2013, 112, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Schon, S.; Klein, A.M.; Ferstl, I.; Kizgin, A.; Tannich, E.; Kuhn, C.; Pluskota, B.; Jost, A. First mass development of Aedes albopictus (Diptera: Culicidae)-its surveillance and control in Germany. Parasitol. Res. 2017, 116, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Pluskota, B.; Jöst, A.; Augsten, X.; Stelzner, L.; Ferstl, I.; Becker, N. Successful overwintering of Aedes albopictus in Germany. Parasitol. Res. 2016, 115, 3245–3247. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.; Scheuch, D.E.; Kampen, H. The invasive Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in Germany: Local reproduction and overwintering. Acta Trop. 2017, 166, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Fauci, A.S. Chikungunya at the door-deja vu all over again? N. Engl. J. Med. 2014, 371, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.C.; Vazquez-Calvo, A.; Blazquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martin-Acebes, M.A. Zika virus: The latest newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Oxley, D.; Jain, C. Global Air Passenger Markets: Riding out Periods of Turbulence. Available online: http://www.iata.org/publications/economic-briefings/WEF_TTCR_Chapter1.4_2015.pdf (accessed on 20 April 2018).
- German Statistical Office: Air Travel Statistics (Table ID: 46421-0007). Available online: https://www-genesis.destatis.de/genesis/online/data?operation=abruftabelleAbrufen&selectionname=46421-0007 (accessed on 20 April 2018).
- Semenza, J.C.; Sudre, B.; Miniota, J.; Rossi, M.; Hu, W.; Kossowsky, D.; Suk, J.E.; Van Bortel, W.; Khan, K. International dispersal of dengue through air travel: Importation risk for europe. PLoS Negl. Trop. Dis. 2014, 8, e3278. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, N.B.; Caminade, C.; Beierkuhnlein, C.; Thomas, S.M. Mosquito-borne diseases: Advances in modelling climate-change impacts. Trends Parasitol. 2018, 34, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Adhami, J.; Reiter, P. Introduction and establishment of Aedes (Stegomyia) albopictus Skuse (Diptera: Culicidae) in Albania. J. Am. Mosq. Contr. Assoc. 1998, 14, 340–343. [Google Scholar]
- European Centre for Disease Prevention and Control. Mosquito Maps. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps (accessed on 2 May 2018).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.; Mac Nally, R. R Package Version 1.0-4: Hier. Part: Hierarchical Partitioning; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble platform for species distribution modeling. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- GCM Downscaled Data Portal. Available online: http://ccafs-climate.org/ (accessed on 2 May 2018).
- IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Intergovermental Panel on Climate Change: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Chang. 2011, 109. [Google Scholar] [CrossRef]
- Bundesministerium der Justiz und für Verbraucherschutz Verordnung zur Anpassung der Meldepflichten nach dem Infektionsschutzgesetz an die epidemische Lage. In Bundesgesetzblatt Teil I Nr. 13 vom 31. März 2016, IfSG-Meldepflicht-Anpassungsverordnung; 2016; Available online: https://www.gesetze-im-internet.de/ifsgmeldanpv/index.html (accessed on 2 May 2018).
- SurvNet. Available online: http://www.rki.de/DE/Content/Infekt/IfSG/Software/software_inhalt.html (accessed on 2 May 2018).
- Verwaltungsgebiete Mit Einwohnerzahlen 1:250 000—Stand 31.12.2016. Available online: http://www.geodatenzentrum.de/geodaten/gdz_rahmen.gdz_div?gdz_spr=deu&gdz_akt_zeile=5&gdz_anz_zeile=1&gdz_unt_zeile=15&gdz_user_id=0 (accessed on 2 May 2018).
- Kampen, H.; Schuhbauer, A.; Walther, D. Emerging mosquito species in Germany-a synopsis after 6 years of mosquito monitoring (2011–2016). Parasitol. Res. 2017, 116, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Halasa, Y.A.; Shepard, D.S.; Fonseca, D.M.; Farajollahi, A.; Healy, S.; Gaugler, R.; Bartlett-Healy, K.; Strickman, D.A.; Clark, G.G. Quantifying the impact of mosquitoes on quality of life and enjoyment of yard and porch activities in New Jersey. PLoS ONE 2014, 9, e89221. [Google Scholar] [CrossRef] [PubMed]
- Bittner, T.; Jaeschke, A.; Reineking, B.; Beierkuhnlein, C. Comparing modelling approaches at two levels of biological organisation—Climate change impacts on selected Natura 2000 habitats. J. Veg. Sci. 2011, 22, 699–710. [Google Scholar] [CrossRef]
- Expertenkommission Stechmücken als Uberträger von Krankheitserregern. Aedes albopictus in Deutschland—Handlungsbedarf und-optionen im Umgang mit der Asiatischen Tigermücke. Available online: https://www.fli.de/fileadmin/FLI/Publikationen/Handlungsempfehlung/Handlungsempfehlung_Aedes-albopictus_2016-04-19.pdf (accessed on 19 April 2018).
- Dormann, C.F.; Purschke, O.; García Márquez, J.R.; Lautenbach, S.; Schröder, B. Components of uncertainty in species distribution analysis: A case study of the Great Grey Shrike. Ecology 2008, 89, 3371–3386. [Google Scholar] [CrossRef] [PubMed]
- VanDerWal, J.; Shoo, L.P.; Graham, C.; Williams, S.E. Selecting pseudo-absence data for presence-only distribution modeling: How far should you stray from what you know? Ecol. Mod. 2009, 220, 589–594. [Google Scholar] [CrossRef]
- Medley, K. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010, 19, 122–133. [Google Scholar] [CrossRef]
- Tjaden, N.B.; Thomas, S.M.; Fischer, D.; Beierkuhnlein, C. Extrinsic incubation period of dengue: Knowledge, backlog, and applications of temperature dependence. PLoS Negl. Trop. Dis. 2013, 7, e2207. [Google Scholar] [CrossRef] [PubMed]

| Virus | Year | Region | Number of Cases | Reference |
|---|---|---|---|---|
| CHIKV | 2007 | Ravenna region, Italy | ca. 200 | [12] |
| CHIKV | 2010 | Var, France | 2 | [13] |
| CHIKV | 2014 | Montpellier, France | 14 | [14] |
| CHIKV | 2017 | Var, France | 9 | [15] |
| CHIKV | 2017 | Rome and Anzio, Italy | ca. 400 | [16] |
| DENV | 2010 | Nice, France | 2 | [17] |
| DENV | 2010 | Croatia | 1 | [18] |
| DENV | 2013 | Bouches-du-Rhône, France | 1 | [19] |
| DENV | 2015 | Nîmes, France | 7 | [20] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.M.; Tjaden, N.B.; Frank, C.; Jaeschke, A.; Zipfel, L.; Wagner-Wiening, C.; Faber, M.; Beierkuhnlein, C.; Stark, K. Areas with High Hazard Potential for Autochthonous Transmission of Aedes albopictus-Associated Arboviruses in Germany. Int. J. Environ. Res. Public Health 2018, 15, 1270. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15061270
Thomas SM, Tjaden NB, Frank C, Jaeschke A, Zipfel L, Wagner-Wiening C, Faber M, Beierkuhnlein C, Stark K. Areas with High Hazard Potential for Autochthonous Transmission of Aedes albopictus-Associated Arboviruses in Germany. International Journal of Environmental Research and Public Health. 2018; 15(6):1270. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15061270
Chicago/Turabian StyleThomas, Stephanie Margarete, Nils Benjamin Tjaden, Christina Frank, Anja Jaeschke, Lukas Zipfel, Christiane Wagner-Wiening, Mirko Faber, Carl Beierkuhnlein, and Klaus Stark. 2018. "Areas with High Hazard Potential for Autochthonous Transmission of Aedes albopictus-Associated Arboviruses in Germany" International Journal of Environmental Research and Public Health 15, no. 6: 1270. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15061270






