Risk Assessment and Mapping of Hand, Foot, and Mouth Disease at the County Level in Mainland China Using Spatiotemporal Zero-Inflated Bayesian Hierarchical Models
Abstract
:1. Introduction
2. Materials and Methods
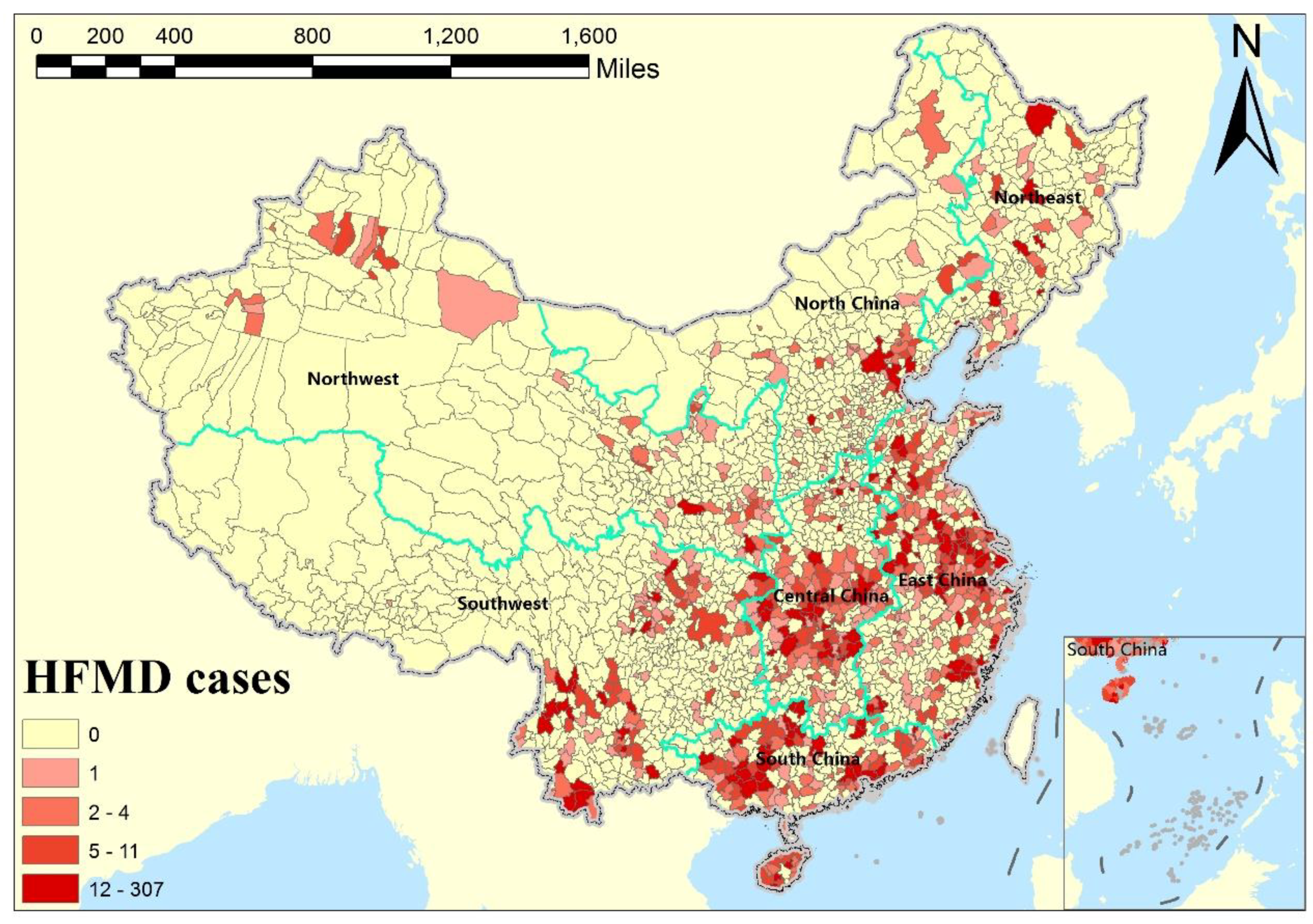
2.1. Data and Study Area
2.2. Statistical Models
2.2.1. Spatiotemporal Epidemic Models
2.2.2. Zero-Inflated Models
2.2.3. Spatiotemporal Zero-Inflated Models
2.2.4. Covariates Selection
2.3. Model Evaluation Methods
2.3.1. Deviance Information Criterion
2.3.2. Conditional Predictive Ordinate
2.3.3. Watanabe-Akaike Information Criterion
2.4. Model Inference
3. Results
3.1. Model Evaluation and Comparison
3.2. Environmental Risk Factors for HFMD
3.3. Temporal Risk Effects of HFMD
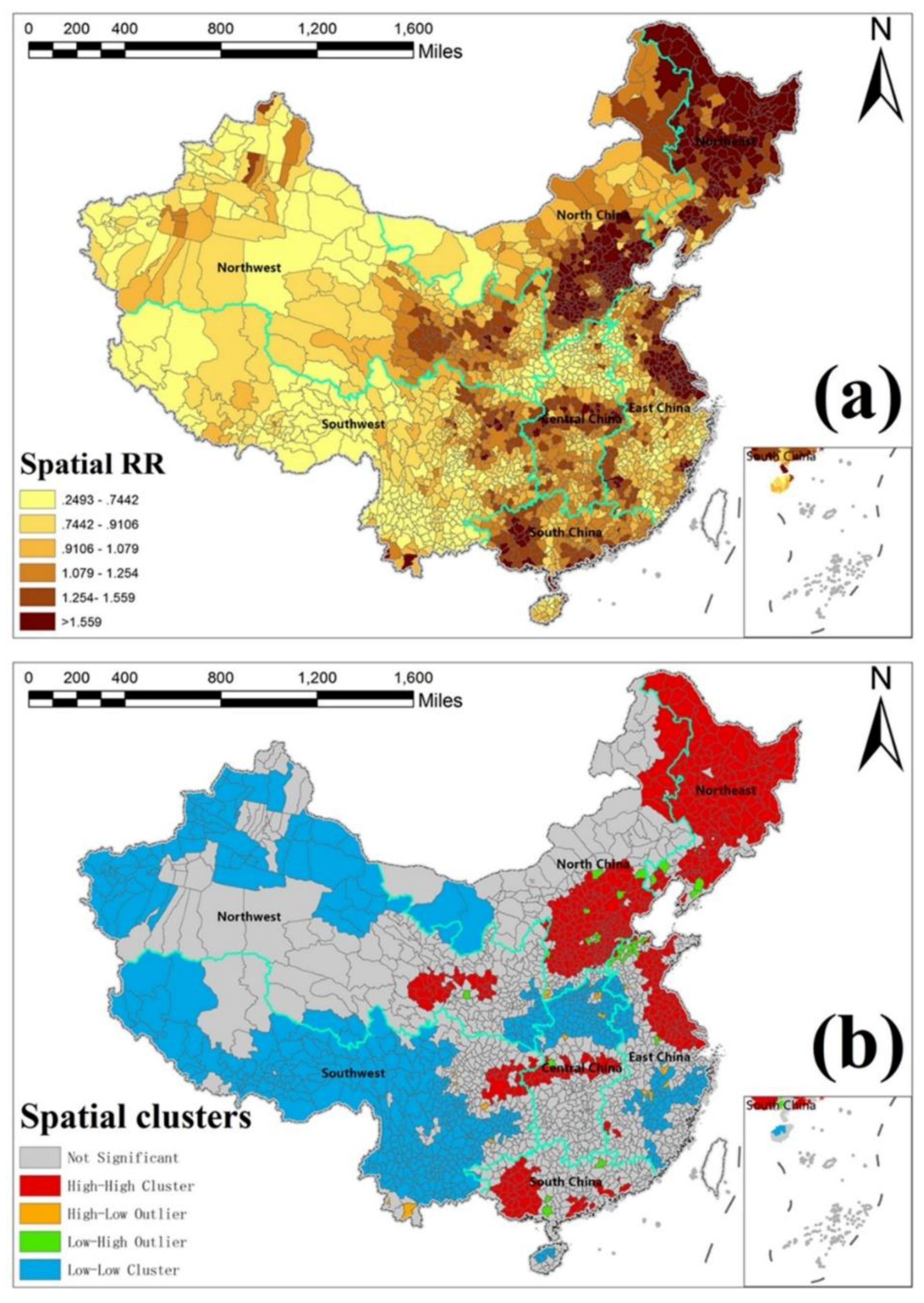
3.4. Spatially Risk Effects of HFMD
3.5. Estimated Spatiotemporal SMR Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koh, W.M.; Bogich, T.; Siegel, K.; Jin, J.; Chong, E.Y.; Tan, C.Y.; Chen, M.I.; Horby, P.; Cook, A.R. The epidemiology of hand, foot and mouth disease in Asia: A systematic review and analysis. Pediatr. Infect. Dis. J. 2016, 35, e285. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.; Chang, Z.; Liu, F.; Fang, V.; Zheng, Y. Hand, foot, and mouth disease in China, 2008–2012: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef]
- Liu, S.L.; Pan, H.; Liu, P.; Amer, S.; Chan, T.C.; Zhan, J.; Huo, X.; Liu, Y.; Teng, Z.; Wang, L. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev. Med. Virol. 2015, 25, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, Z.; Wang, X.; Li, J.; Wang, L.; Liu, Y.; Xue, F.; Liu, Y. The impact of ambient temperature on childhood HFMD incidence in inland and coastal area: A two-city study in shandong province, China. Int. J. Environ. Res. Public Health 2015, 12, 8691–8704. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Gasparrini, A.; Huang, J.; Liao, Q.; Liu, F.; Yin, F.; Yu, H.; Li, X. The exposure-response relationship between temperature and childhood hand, foot and mouth disease: A multicity study from mainland China. Environ. Int. 2017, 100, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, D.; Hashizume, M. The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci. Total Environ. 2011, 410, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Bai, L.; Zhang, Y.; Zhang, H.; Wang, S.; Xie, M.; Zhao, D.; Su, H. Ambient temperature, humidity and hand, foot, and mouth disease: A systematic review and meta-analysis. Sci. Total Environ. 2018, 625, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Christakos, G.; Yang, W.; Liao, Y.; Zhong, J.; Li, X.; Lai, S.; Chen, H. Hand, foot and mouth disease: Spatiotemporal transmission and climate. Int. J. Health Geogr. 2011, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, H.; Li, X.; Lang, L.; Xiao, X.; Ding, P.; He, P.; Zhang, Y.; Wang, M.; Liu, Q. Short-term effects of meteorological factors on children hand, foot and mouth disease in Guangzhou, China. Int. J. Biometeorol. 2014, 58, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, X.; Chen, X.; Li, Y.; Lu, Y.; Mei, S.; Liao, Y.; Lin, H. Short-term effects of meteorological factors on hand, foot and mouth disease among children in Shenzhen, China: Non-linearity, threshold and interaction. Sci. Total Environ. 2016, 539, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Lam, T.; Wong, C.; Chuang, S. Is hand, foot and mouth disease associated with meteorological parameters? Epidemiol. Infect. 2010, 138, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, Z.; Wang, X.; Liu, Y.; Yuan, Z.; Liu, Y.; Xue, F. Detecting the association between meteorological factors and hand, foot, and mouth disease using spatial panel data models. Int. J. Infect. Dis. 2015, 34, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Z.; Zhang, D.; Yu, S.; Hao, Y. Boosted regression tree model-based assessment of the impacts of meteorological drivers of hand, foot and mouth disease in Guangdong, China. Sci. Total Environ. 2016, 553, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Gray-Donald, K.; O’loughlin, J.; Paradis, G.; Hanley, J. Influence of weather conditions and season on physical activity in adolescents. Ann. Epidemiol. 2009, 19, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, J. Monitoring hand, foot and mouth disease by combining search engine query data and meteorological factors. Sci. Total Environ. 2018, 612, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Song, C.; Wang, J.; Li, X. Using an autologistic regression model to identify spatial risk factors and spatial risk patterns of hand, foot and mouth disease (HFMD) in mainland China. BMC Public Health 2014, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Bo, Y.; Xu, C.; Hu, M.; Huang, D. Identification of health risks of hand, foot and mouth disease in China using the geographical detector technique. Int. J. Environ. Res. Public Health 2014, 11, 3407–3423. [Google Scholar] [CrossRef] [PubMed]
- Xu, C. Spatio-temporal pattern and risk factor analysis of hand, foot and mouth disease associated with under-five morbidity in the Beijing–Tianjin–Hebei region of China. Int. J. Environ. Res. Public Health 2017, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, J.; Guo, Y.; Ou, Q.; Shen, S.; Ou, C.; Liu, Q. Short-term effects of meteorological factors on pediatric hand, foot, and mouth disease in Guangdong, China: A multi-city time-series analysis. BMC Infect. Dis. 2016, 16, 524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Pang, C.; Yuan, Z.; Li, H.; Xue, F. Spatio-temporal analysis of the relationship between climate and hand, foot, and mouth disease in Shandong province, China, 2008–2012. BMC Infect. Dis. 2015, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zeng, D.; Wang, Q.; Zheng, X.; Wang, F. An epidemiological analysis of the Beijing 2008 Hand-Foot-Mouth epidemic. Chin. Sci. Bull. 2010, 55, 1142–1149. [Google Scholar] [CrossRef]
- Liao, J.; Qin, Z.; Zuo, Z.; Yu, S.; Zhang, J. Spatial-temporal mapping of hand foot and mouth disease and the long-term effects associated with climate and socio-economic variables in Sichuan province, China from 2009 to 2013. Sci. Total Environ. 2016, 563, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, C.; Tong, S.; Chen, H.; Yang, W. Spatial dynamic patterns of hand-foot-mouth disease in the people’s republic of China. Geospat. Health 2013, 7, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Zhang, Y.; Xu, Q.; Huang, F.; Cao, K.; Tao, L.; Guo, J.; Gao, Q.; Wang, W. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the county level in mainland China, 2008–2012. PLoS ONE 2016, 11, e0147532. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, J.; Xu, C.; Lai, S.; Yang, W. Spatiotemporal pattern of hand–foot–mouth disease in China: An analysis of empirical orthogonal functions. Public Health 2014, 128, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Arab, A. Spatial and spatio-temporal models for modeling epidemiological data with excess zeros. Int. J. Environ. Res. Public Health 2015, 12, 10536–10548. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, B.; Fan, J.; Wang, F.; Liu, Q. A study of the dengue epidemic and meteorological factors in Guangzhou, China, by using a zero-inflated poisson regression model. Asia Pac. J. Public Health 2014, 26, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Amek, N.; Bayoh, N.; Hamel, M.; Lindblade, K.A.; Gimnig, J.; Laserson, K.F.; Slutsker, L.; Smith, T.; Vounatsou, P. Spatio-temporal modeling of sparse geostatistical malaria sporozoite rate data using a zero inflated binomial model. Spat. Spat.-Tempor. Epidemiol. 2011, 2, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Musenge, E.; Vounatsou, P.; Kahn, K. Space-time confounding adjusted determinants of child HIV/TB mortality for large zero-inflated data in rural South Africa. Spat. Spat.-Tempor. Epidemiol. 2011, 2, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Musal, M.; Aktekin, T. Bayesian spatial modeling of HIV mortality via zero-inflated poisson models. Stat. Med. 2013, 32, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, J.D.; Thomson, W.M. The utility of the zero-inflated poisson and zero-inflated negative binomial models: A case study of cross-sectional and longitudinal dmf data examining the effect of socio-economic status. Commun. Dent. Oral Epidemiol. 2004, 32, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.B. Zero-inflated models for regression analysis of count data: A study of growth and development. Stat. Med. 2002, 21, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Zero-Truncated and Zero-Inflated Models for Count Data; Springer: New York, NY, USA, 2009; pp. 261–293. [Google Scholar]
- Blangiardo, M.; Cameletti, M.; Baio, G.; Rue, H. Spatial and spatio-temporal models with R-INLA. Spat. Spat.-Tempor. Epidemiol. 2013, 4, 33–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrödle, B.; Held, L. Spatio-temporal disease mapping using INLA. Environmetrics 2011, 22, 725–734. [Google Scholar] [CrossRef]
- Musio, M.; Sauleau, E.A.; Buemi, A. Bayesian semi-parametric zip models with space–time interactions: An application to cancer registry data. Math. Med. Biol. J. IMA 2010, 27, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Barber, X.; Conesa, D.; López-Quílez, A.; Mayoral, A.; Morales, J.; Barber, A. Bayesian hierarchical models for analysing the spatial distribution of bioclimatic indices. SORT-Stat. Oper. Res. Trans. 2017, 1, 277–296. [Google Scholar]
- Gracia, E.; López-Quílez, A.; Marco, M.; Lladosa, S.; Lila, M. Exploring neighborhood influences on small-area variations in intimate partner violence risk: A bayesian random-effects modeling approach. Int. J. Environ. Res. Public Health 2014, 11, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, X.; Shi, X.; Bo, Y.; Wang, J. Estimating missing values in China’s official socioeconomic statistics using progressive spatiotemporal bayesian hierarchical modeling. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Knorr-Held, L.; Raßer, G. Bayesian detection of clusters and discontinuities in disease maps. Biometrics 2000, 56, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.B.; Clark, A. Spatial mixture relative risk models applied to disease mapping. Stat. Med. 2002, 21, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kai, F. What’s the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef] [PubMed]
- Besag, J. Spatial interaction and the statistical analysis of lattice systems. J. R. Stat. Soc. Ser. B (Methodol.) 1974, 36, 192–236. [Google Scholar]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Rue, H.; Held, L. Gaussian Markov Random Fields: Theory and Applications; CRC Press: Boca Raton, FL, USA, 2005; Chapter 3; p. 95. [Google Scholar]
- Lambert, D. Zero-inflated poisson regression, with an application to defects in manufacturing. Technometrics 1992, 34, 1–14. [Google Scholar] [CrossRef]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology (Sunnyvale Calif.) 2016, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2002, 64, 583–639. [Google Scholar] [CrossRef] [Green Version]
- Gneiting, T.; Raftery, A.E. Strictly proper scoring rules, prediction, and estimation. J. Am. Stat. Assoc. 2007, 102, 359–378. [Google Scholar] [CrossRef]
- Watanabe, S. Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory. J. Mach. Learn. Res. 2010, 11, 3571–3594. [Google Scholar]
- Vehtari, A.; Gelman, A.; Gabry, J. Practical bayesian model evaluation using leave-one-out cross-validation and waic. Stat. Comput. 2017, 27, 1413–1432. [Google Scholar] [CrossRef]
- Lindgren, F.; Rue, H. Bayesian spatial modelling with R-INLA. J. Stat. Softw. 2015, 63, 19. [Google Scholar] [CrossRef]
- Ugarte, M.D.; Adin, A.; Goicoa, T.; Militino, A.F. On fitting spatio-temporal disease mapping models using approximate bayesian inference. Stat. Methods Med. Res. 2014, 23, 507–530. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, W.; Xu, C.; Wang, J. A spatiotemporal mixed model to assess the influence of environmental and socioeconomic factors on the incidence of hand, foot and mouth disease. BMC Public Health 2018, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Pu, D.; Mo, X.; Zhu, C.; Gong, S.; Xu, Y.; Lin, G.; Wu, B.; He, S.; Jiao, X. Children of rural-to-urban migrant workers in China are at a higher risk of contracting severe hand, foot and mouth disease and ev71 infection: A hospital-based study. Emerg. Microbes Infect. 2013, 2, e72. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, G.; Zheng, S.; Cheng, J.; Lei, G.; Tian, K.; Wu, Y.; Xie, X.; Xu, M.; Ji, W. Research on the Environmental Impact Factors of Hand-Foot-Mouth Disease in Shenzhen, China Using RS and GIS Technologies. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; pp. 7240–7243. [Google Scholar]
- Hu, M.; Li, Z.; Wang, J.; Jia, L.; Liao, Y.; Lai, S.; Guo, Y.; Zhao, D.; Yang, W. Determinants of the incidence of hand, foot and mouth disease in China using geographically weighted regression models. PLoS ONE 2012, 7, e38978. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhang, X. Modeling and preventive measures of hand, foot and mouth disease (HFMD) in China. Int. J. Environ. Res. Public Health 2014, 11, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bo, Y.; de Jong, R.; Li, A.; Zhu, Y.; Cheng, J. Comparison of vegetation phenological metrics extracted from gimms ndvig and meris mtci data sets over China. Int. J. Remote Sens. 2015, 36, 300–317. [Google Scholar] [CrossRef]
- Monod, A. Generalized estimating equations for zero-inflated spatial count data. Procedia Environ. Sci. 2011, 7, 281–286. [Google Scholar] [CrossRef]
- Malesios, C.; Demiris, N.; Kostoulas, P.; Dadousis, K.; Koutroumanidis, T.; Abas, Z. Spatio-Temporal Modeling of Foot-and-Mouth Outbreaks (24 August 2017). Available online: http://0-dx-doi-org.brum.beds.ac.uk/10.2139/ssrn.3025787 (accessed on 11 July 2018).
- Ren, Z.; Zhu, J.; Gao, Y.; Yin, Q.; Hu, M.; Dai, L.; Deng, C.; Yi, L.; Deng, K.; Wang, Y. Maternal exposure to ambient PM 10 during pregnancy increases the risk of congenital heart defects: Evidence from machine learning models. Sci. Total Environ. 2018, 630, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, L.; Song, C.; Yin, H.; Yang, J. Spatiotemporal assessment of PM 2.5-related economic losses from health impacts during 2014–2016 in China. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed]


| Model | DIC | PD | LS | WAIC | PW |
|---|---|---|---|---|---|
| model 1 | 352883 | 2112 | 7.19 | 381936 | 27738 |
| model 2 | 317909 | 1983 | 6.55 | 343243 | 24113 |
| model 3 | 152998 | 1948 | 2.94 | 153404 | 2022 |
| model 4 | 151201 | 1934 | 2.87 | 151543 | 1982 |
| Variables Name | Mean | 0.025 CI | 0.975 CI | SD | RR |
|---|---|---|---|---|---|
| Temperature | 0.7053 | 0.6664 | 0.7441 | 0.0198 | 2.02 |
| Relative humidity | 0.1112 | 0.0682 | 0.1542 | 0.0219 | 1.12 |
| Wind speed | 0.2150 | 0.1791 | 0.2510 | 0.0183 | 1.24 |
| Sunshine hours | 0.1444 | 0.1017 | 0.1871 | 0.0218 | 1.16 |
| Proportion of children | 0.1344 | 0.0208 | 0.2480 | 0.0579 | 1.14 |
| Enterprise number density | 0.3406 | 0.2180 | 0.4631 | 0.0624 | 1.41 |
| Per capita gross domestic product (GDP) | 0.1970 | 0.0841 | 0.3098 | 0.0575 | 1.22 |
| Per capita fixed assets investment | 0.3637 | 0.2517 | 0.4755 | 0.0570 | 1.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; He, Y.; Bo, Y.; Wang, J.; Ren, Z.; Yang, H. Risk Assessment and Mapping of Hand, Foot, and Mouth Disease at the County Level in Mainland China Using Spatiotemporal Zero-Inflated Bayesian Hierarchical Models. Int. J. Environ. Res. Public Health 2018, 15, 1476. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15071476
Song C, He Y, Bo Y, Wang J, Ren Z, Yang H. Risk Assessment and Mapping of Hand, Foot, and Mouth Disease at the County Level in Mainland China Using Spatiotemporal Zero-Inflated Bayesian Hierarchical Models. International Journal of Environmental Research and Public Health. 2018; 15(7):1476. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15071476
Chicago/Turabian StyleSong, Chao, Yaqian He, Yanchen Bo, Jinfeng Wang, Zhoupeng Ren, and Huibin Yang. 2018. "Risk Assessment and Mapping of Hand, Foot, and Mouth Disease at the County Level in Mainland China Using Spatiotemporal Zero-Inflated Bayesian Hierarchical Models" International Journal of Environmental Research and Public Health 15, no. 7: 1476. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15071476





