Methylmercury in Industrial Harbor Sediments in Taiwan: First Observations on Its Occurrence, Distribution, and Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Collection and Analysis
2.2. Methylmercury Analysis in Sediment
2.2.1. Chemicals and Reagents
2.2.2. MeHg Analytical Procedure
2.2.3. GC-MS Instrumentation and Conditions
2.2.4. Identification and Quantification of MeHg
2.3. Data Analysis
3. Results and Discussion
3.1. Quality Assurance and Quality Control (QAQC) for Methylmercury Analysis
3.2. GC-MS Separation and Identification
3.3. Sediment Characteristics
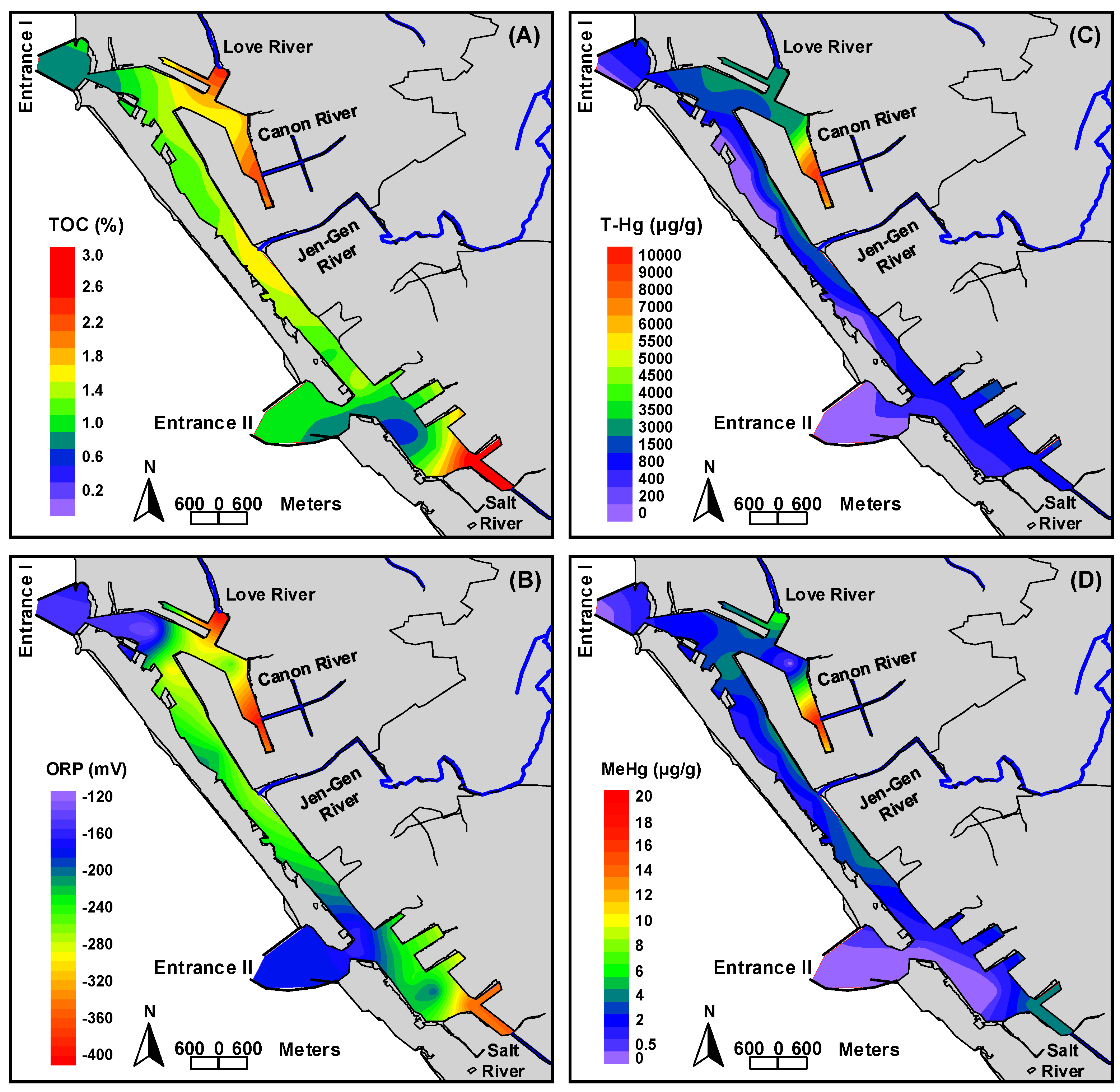
3.4. Distribution of Total Mercury and Methylmercury in Sediments
3.5. Correlation of Sediment Characteristics and Total Mercury and Methylmercury
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wong, M.H. Environmental mercury contamination in China: Sources and impacts. Environ. Int. 2007, 33, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Bigham, G.N.; Murray, K.J.; Masue-Slowey, Y.; Henry, E.A. Biogeochemical controls on methylmercury in soils and sediments: Implications for site management. Integr. Environ. Assess. Manag. 2017, 13, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Strickman, R.J.; Mitchell, C.P.J. Methylmercury production and accumulation in urban stormwater ponds and habitat wetlands. Environ. Pollut. 2017, 221, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Podar, M.; Bullock, A.L.; Graham, A.M.; Brown, S.D.; Somenahally, A.C.; Johs, A.; Hurt, R.A.; Bailey, K.L.; Elias, D.A. Mercury methylation by novel microorganisms from new environments. Environ. Sci. Technol. 2013, 47, 11810–11820. [Google Scholar] [CrossRef] [PubMed]
- Kerin, E.J.; Gilmour, C.C.; Roden, E.; Suzuki, M.T.; Coates, J.D.; Mason, R.P. Mercury methylation by dissimilatory iron-reducing bacteria. Appl. Environ. Microbiol. 2006, 72, 7919–7921. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, S.; Amyot, M.; Barkay, T.; Wang, Y.P.; Planas, D. Methanogens: Principal methylators of mercury in lake periphyton. Environ. Sci. Technol. 2011, 45, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Q.; Reinfelder, J.R.; Hines, M.E.; Barkay, T. Mercury methylation by the methanogen Methanospirillum hungatei. Appl. Environ. Microbiol. 2013, 79, 6325–6330. [Google Scholar] [CrossRef] [PubMed]
- Schartup, A.T.; Mason, R.P.; Balcom, P.H.; Hollweg, T.A.; Chen, C.Y. Methylmercury production in estuarine sediments: Role of organic matter. Environ. Sci. Technol. 2013, 47, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; Vassileva, E. Determination of methylmercury in marine sediment samples: Method validation and occurrence data. Anal. Chim. Acta 2015, 853, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: A critical review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Smith, R.G., Jr.; Lee, R.F.; Windom, H.L.; Heitmuller, P.T.; Macauley, J.M.; Summers, J.K. Distribution of total mercury and methylmercury in water sediment and fish from south Florida estuaries. Arch. Environ. Contam. Toxicol. 1998, 34, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Haris, H.; Aris, A.Z.; Mokhtar, M. Mercury and methylmercury distribution in the intertidal surface sediment of a heavily anthrophogenically impacted saltwater-mangrove-sediment interplay zone. Chemosphere 2017, 166, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chou, S.S.; Hwang, D.F. Determination of methyl- and inorganic mercury in fish using focused microwave digestion followed by Cu++ addition, sodium tetraethylborate derivatization, n-Heptane extraction, and gas chromatography mass spectrometry. J. Food Drug. Anal. 2004, 12, 175–182. [Google Scholar]
- Chen, C.Y.; Ward, D.M.; Williams, J.J.; Fisher, N.S. Metal bioaccumulation by estuarine food webs in New England, USA. J. Mar. Sci. Eng. 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.W.; Chen, C.F. Distribution, enrichment, accumulation and potential ecological risks of mercury in the sediments of Kaohsiung Harbor, Taiwan. Chem. Ecol. 2013, 29, 693–708. [Google Scholar] [CrossRef]
- Loring, D.H.; Rantala, R.T.T. Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth-Sci. Rev. 1992, 32, 235–283. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, C.F.; Dong, C.D.; Tu, Y.T. Composition and source apportionment of PAHs in sediments at river mouths and channel in Kaohsiung Harbor, Taiwan. J. Environ. Monit. 2012, 14, 105–115. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Analysis. Soil Acid-Base Value (pH) Determination Method—Electrode Method, NIEA S410.62C, Taiwan. 2009. Available online: http://www.taiwanlab.com.tw/protocol/show.asp?id=1407 (accessed on 15 August 2018).
- National Institute of Environmental Analysis. Sediment Sampling Methods, NIEA S104.32B, Taiwan. 2016. Available online: http://www.rootlaw.com.tw/LawContent.aspx?LawID=A040300081076900-1050104 (accessed on 15 August 2018).
- Long, E.R.; MacDonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–87. [Google Scholar] [CrossRef]
- Chen, C.F.; Dong, C.D.; Chen, C.W. Metal speciation and contamination in dredged harbor sediments from Kaohsiung Harbor, Taiwan. Soil. Sediment. Contam. 2013, 22, 546–561. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, Y.; Liu, X.; Kuang, W.; Yuang, D.; Ji, W. Environmental impact factors and mercury speciation in the sediment along Fujian and eastern Guangdong coasts. Acta Oceanol. Sin. 2013, 32, 76–80. [Google Scholar] [CrossRef]
- Bełdowski, J.; Miotk, M.; Bełdowska, M.; Pempkowiak, J. Total, methyl and organic mercury in sediments of the Southern Baltic Sea. Mar. Pollut. Bull. 2014, 87, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Yano, S.; Hisano, A.; Kindaichi, M.; Sonoda, I.; Tada, A.; Akagi, H. Distribution and characteristics of methylmercury in surface sediment in Minamata Bay. Mar. Pollut. Bull. 2016, 109, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Lawrence, A.L. Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay Maryland, USA. Environ. Toxicol. Chem. 1999, 18, 2438–2447. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the aquatic environment: A review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Heyes, A.; Miller, C.; Mason, R.P. Mercury and methylmercury in Hudson River sediment impact of tidal resuspension on partitioning and methylation. Mar. Chem. 2004, 90, 75–89. [Google Scholar] [CrossRef]
- Conaway, C.H.; Squire, S.; Mason, R.P.; Flegal, A.R. Mercury speciation in the San Francisco Bay estuary. Mar. Chem. 2003, 80, 199–225. [Google Scholar] [CrossRef]
- Taylor, D.L.; Linehan, J.C.; Murray, D.W.; Prell, W.L. Indicators of sediment and biotic mercury contamination in a southern New England estuary. Mar. Pollut. Bull. 2012, 64, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic Sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Spada, L.; Annicchiarico, C.; Cardellicchio, N.; Giandomenico, S.; Di Leo, A. Mercury and methylmercury concentrations in Mediterranean seafood and surface sediments, intake evaluation and risk for consumers. Int. J. Hyg. Environ. Health. 2012, 215, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Falandysz, J. Speciation and concentrations of mercury in certain coastal marine sediments. Water Air Soil Pollut. 1998, 103, 129–136. [Google Scholar] [CrossRef]
- Ogrinc, N.; Monperrus, M.; Kotnika, J.; Fajona, V.; Vidimovab, K.; Amouroux, D.; Kocmana, D.; Tessier, E.; Žižek, S.; Horvat, M. Distribution of mercury and methylmercury in deep-sea surficial sediments of Mediterranean Sea. Mar. Chem. 2007, 107, 31–48. [Google Scholar] [CrossRef]
- Landaluze, J.S.; De Diego, A.; Raposo, J.C.; Madariaga, J.M. Methylmercury determination in sediments and fish tissues from the Nerbioi-Ibaizabal estuary (Basque Country, Spain). Anal. Chim. Acta 2004, 508, 107–117. [Google Scholar] [CrossRef]
- Agah, H.; Elskens, M.; Fatemi, S.M.R.; Owfi, F.; Baeyens, W.; Leermakers, M. Mercury speciation in the Persian Gulf sediments. Environ. Monit. Assess. 2009, 157, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Agah, H.; Owfi, F.; Sharif Fazeli, M.; Fatemi, S.M.R.; Savari, A. Determining mercury and methylmercury in sediments of the northern parts of the Persian Gulf. J. Oceanogr. 2010, 1, 7–13. [Google Scholar]
- Abi-Ghanem, C.; Nakhlé, K.; Khalaf, G.; Cossa, D. Mercury distribution and methylmercury mobility in the sediments of three sites on the Lebanese coast, eastern Mediterranean. Arch. Environ. Contam. Toxicol. 2011, 60, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.B.; Ip, C.C.M.; Tang, C.W.Y.; Zhang, G.; Wu, R.S.S.; Li, X.D. Spatial and temporal variations of mercury in sediments from Victoria Harbour, Hong Kong. Mar. Pollut. Bull. 2007, 54, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.B.; Liang, L.N.; Yuan, C.G.; He, B.; Jiang, G.B. Methylmercury and total mercury in sediment collected from the East China Sea. Bull. Environ. Contam. Toxicol. 2005, 74, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.R.; Fitzgerald, W.F. Methylmercury cycling in sediments on the continental shelf of southern New England. Geochim. Cosmochim. Acta 2006, 70, 918–930. [Google Scholar] [CrossRef] [Green Version]
- Randall, P.M.; Chattopadhyay, S. Mercury contaminated sediment sites-an evaluation of remedial options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Kao, C.M.; Chen, C.F.; Dong, C.D. Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 2007, 66, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Feng, X.; Qiu, G.; Li, Z.; Yao, H.; Shang, L.; Yan, H. The impacts of organic matter on the distribution and methylation of mercury in a hydroelectric reservoir in Wujiang River, Southwest China. Environ. Toxicol. Chem. 2016, 35, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Watras, C.J.; Back, R.C.; Halvorsen, S.; Hudson, R.J.M.; Morrison, K.A.; Wente, S.P. Bioaccumulation of mercury in pelagic freshwater food webs. Sci. Total. Environ. 1998, 219, 183–208. [Google Scholar] [CrossRef]
- Fleck, J.A.; Marvin-DiPasquale, M.; Eagles-Smith, C.A.; Ackerman, J.T.; Lutz, M.A.; Tate, M.; Alpers, C.N.; Hall, B.D.; Krabbenhoft, D.P.; Eckley, C.S. Mercury and methylmercury in aquatic sediment across western North America. Sci. Total. Environ. 2016, 568, 727–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Calibration Factor (CF) (n = 6) | Detection Limits (μg/kg dw) | Blank Analysis (n = 6) (μg/kg dw) | Check Analysis (n = 6) R 1 (%) | Duplication Analysis (n = 4) RPD 1 (%) | |
|---|---|---|---|---|---|
| Average ± SD 1 | RSD 1 (%) | ||||
| 2513 ± 103 | 4.1 | 0.31 | <DL | 91.8 ± 6.9 | 6.2 ± 2.4 |
| Compounds | Measured Value (μg/kg dw) | Certified Value (mean ± SD) | |||
|---|---|---|---|---|---|
| #1 | #2 | #3 | Mean ± SD 1 | ||
| MeHg | 77.2 | 72.6 | 73.8 | 74.5 ± 2.4 | 75.5 ± 4.8 |
| Site | Clay | Silt | Sand | TOC | pH | ORP | T-Hg | MeHg | MeHg Ratio 1 |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (mV) | (μg/kg) | (μg/kg) | (%) | ||
| 1 | <0.01 | <0.01 | 100.0 | 0.92 | 7.77 | −146 | 401 | 0.61 | 0.14 |
| 2 | 4.3 | 37.9 | 57.8 | 0.86 | 7.55 | −143 | 702 | 2.49 | 0.33 |
| 3 | 7.5 | 82.9 | 9.6 | 1.33 | 7.31 | −129 | 1699 | 2.55 | 0.14 |
| 4 | 4.2 | 51.7 | 44.1 | 2.50 | 7.32 | −392 | 1988 | 6.81 | 0.32 |
| 5 | 6.2 | 72.9 | 20.9 | 1.63 | 7.40 | −245 | 1944 | <0.31 | 0.01 |
| 6 | 2.3 | 16.2 | 81.5 | 2.39 | 7.31 | −385 | 9035 | 17.67 | 0.18 |
| 7 | 2.2 | 14.3 | 83.5 | 1.53 | 7.50 | −286 | 472 | 4.94 | 0.97 |
| 8 | 7.6 | 60.3 | 32.1 | 1.31 | 7.61 | −252 | 298 | 2.20 | 0.69 |
| 9 | 7.7 | 79.0 | 13.3 | 1.36 | 7.45 | −215 | 297 | 1.99 | 0.62 |
| 10 | 7.1 | 71.2 | 21.8 | 1.72 | 7.43 | −254 | 896 | 2.02 | 0.21 |
| 11 | 4.0 | 35.8 | 60.2 | 1.47 | 7.45 | −222 | 149 | 4.26 | 2.66 |
| 12 | 5.4 | 49.8 | 44.8 | 1.17 | 7.78 | −199 | 374 | 2.33 | 0.58 |
| 13 | 5.5 | 52.1 | 42.4 | 1.52 | 7.74 | −158 | 646 | 1.75 | 0.25 |
| 14 | 8.2 | 69.2 | 22.6 | 1.02 | 7.81 | −220 | 521 | 1.58 | 0.28 |
| 15 | 4.9 | 33.5 | 61.6 | 0.91 | 7.70 | −157 | 223 | <0.31 | 0.06 |
| 16 | 7.4 | 66.9 | 25.8 | 0.71 | 7.58 | −231 | 447 | <0.31 | 0.03 |
| 17 | 7.6 | 69.3 | 23.1 | 1.17 | 7.49 | −193 | 449 | <0.31 | 0.03 |
| 18 | 6.6 | 68.1 | 25.3 | 2.84 | 7.52 | −338 | 524 | 4.19 | 0.75 |
| 19 | 6.4 | 48.7 | 45.0 | 1.01 | 7.65 | −179 | 199 | <0.31 | 0.07 |
| 20 | 6.9 | 61.2 | 31.8 | 1.07 | 7.64 | −174 | 173 | <0.31 | 0.08 |
| Location | T-Hg (μg/kg) | MeHg (μg/kg) | MeHg Ratio (%) | Reference |
|---|---|---|---|---|
| Biscayne Bay, USA | 17 | 0.026 | 0.15 | [11] |
| Tampa Bay, USA | 8.3 | 0.049 | 0.59 | [11] |
| Charlotte Harbor, USA | 29 | 0.074 | 0.26 | [11] |
| Florida Bay, USA | 12 | 0.082 | 0.68 | [11] |
| Pine Island Sound, USA | 6.3 | 0.055 | 0.87 | [11] |
| Hudson river, USA | 1000 | 1.3 | 0.13 | [27] |
| San Francisco Bay, USA | 200 | 0.4 | 0.2 | [28] |
| Narragansett Bay, USA | 555 | 1.852 | 0.42 | [29] |
| Gulf of Trieste, Italy | 5240 | 16.9 | 0.32 | [30] |
| Gulf of Taranto, Italy | 2770 | 10.8 | 0.39 | [31] |
| Vistula Mouth, Poland | 71 | 0.354 | 0.50 | [23] |
| Odra mouth, Poland | 9 | 0.075 | 0.83 | [23] |
| Gulf of Gdansk, Poland | 164 | 0.645 | 0.39 | [32] |
| Anadyr Estuary, Russia | 339 | 0.254 | 0.22 | [32] |
| Mediterranean Basin | 110 | 1.33 | 1.21 | [33] |
| Nerbion-Ibaizabal Estuary, Spain | 2005 | 46.8 | 2.33 | [34] |
| Taheri Port, Iran | 34 | 0.2 | 0.69 | [35] |
| Dayyer Port, Iran | 26 | 0.2 | 0.65 | [35] |
| Persian Gulf, Iran | 20 | 0.14 | 0.71 | [36] |
| Dora, Lebanon | 100–650 | 0.07–0.5 | 0.03–0.35 | [37] |
| Minamata Bay, Japan | 2960 | 1.74 | 0.06 | [24] |
| Isahaya Bay, Japan | 66 | 0.11 | 0.16 | [24] |
| Off Kuala Terengganu, Malaysia | 61 | 0.038 | 0.11 | [32] |
| Victoria Harbor, Hong Kong | 247 | <0.1–1.5 | 0.14 | [38] |
| East China Sea, China | 37 | 2.7 | 8.8 | [39] |
| Fujian and Guangdong coasts, China | 31.5 | 0.096 | 0.30 | [22] |
| Kaohsiung Harbor, Taiwan | 1072 | 2.86 | 0.42 | This study |
| Item | Clay | Silt | Sand | TOC | pH | ORP | T-Hg | MeHg |
|---|---|---|---|---|---|---|---|---|
| Silt | 0.939 a | |||||||
| Sand | −0.949 a | −1.000 a | ||||||
| TOC | −0.140 | −0.032 | 0.017 | |||||
| pH | 0.000 | −0.198 | 0.181 | −0.571 a | ||||
| ORP | 0.159 | 0.077 | −0.085 | −0.828 a | 0.515 b | |||
| T-Hg | −0.329 | −0.267 | 0.274 | 0.500 b | −0.510 b | −0.553 b | ||
| MeHg | −0.441 | −0.392 | 0.398 | 0.653 a | −0.511 b | −0.711 a | 0.889 a | |
| MeHg ratio | −0.199 | −0.199 | 0.220 | 0.177 | −0.129 | −0.154 | −0.171 | 0.172 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-F.; Ju, Y.-R.; Lin, G.-T.; Chen, C.-W.; Dong, C.-D. Methylmercury in Industrial Harbor Sediments in Taiwan: First Observations on Its Occurrence, Distribution, and Measurement. Int. J. Environ. Res. Public Health 2018, 15, 1765. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15081765
Chen C-F, Ju Y-R, Lin G-T, Chen C-W, Dong C-D. Methylmercury in Industrial Harbor Sediments in Taiwan: First Observations on Its Occurrence, Distribution, and Measurement. International Journal of Environmental Research and Public Health. 2018; 15(8):1765. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15081765
Chicago/Turabian StyleChen, Chih-Feng, Yun-Ru Ju, Guan-Ting Lin, Chiu-Wen Chen, and Cheng-Di Dong. 2018. "Methylmercury in Industrial Harbor Sediments in Taiwan: First Observations on Its Occurrence, Distribution, and Measurement" International Journal of Environmental Research and Public Health 15, no. 8: 1765. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15081765




