Lead in Air in Bangladesh: Exposure in a Rural Community with Elevated Blood Lead Concentrations among Young Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Measurements
2.2. Environmental Sample Collection and Analysis
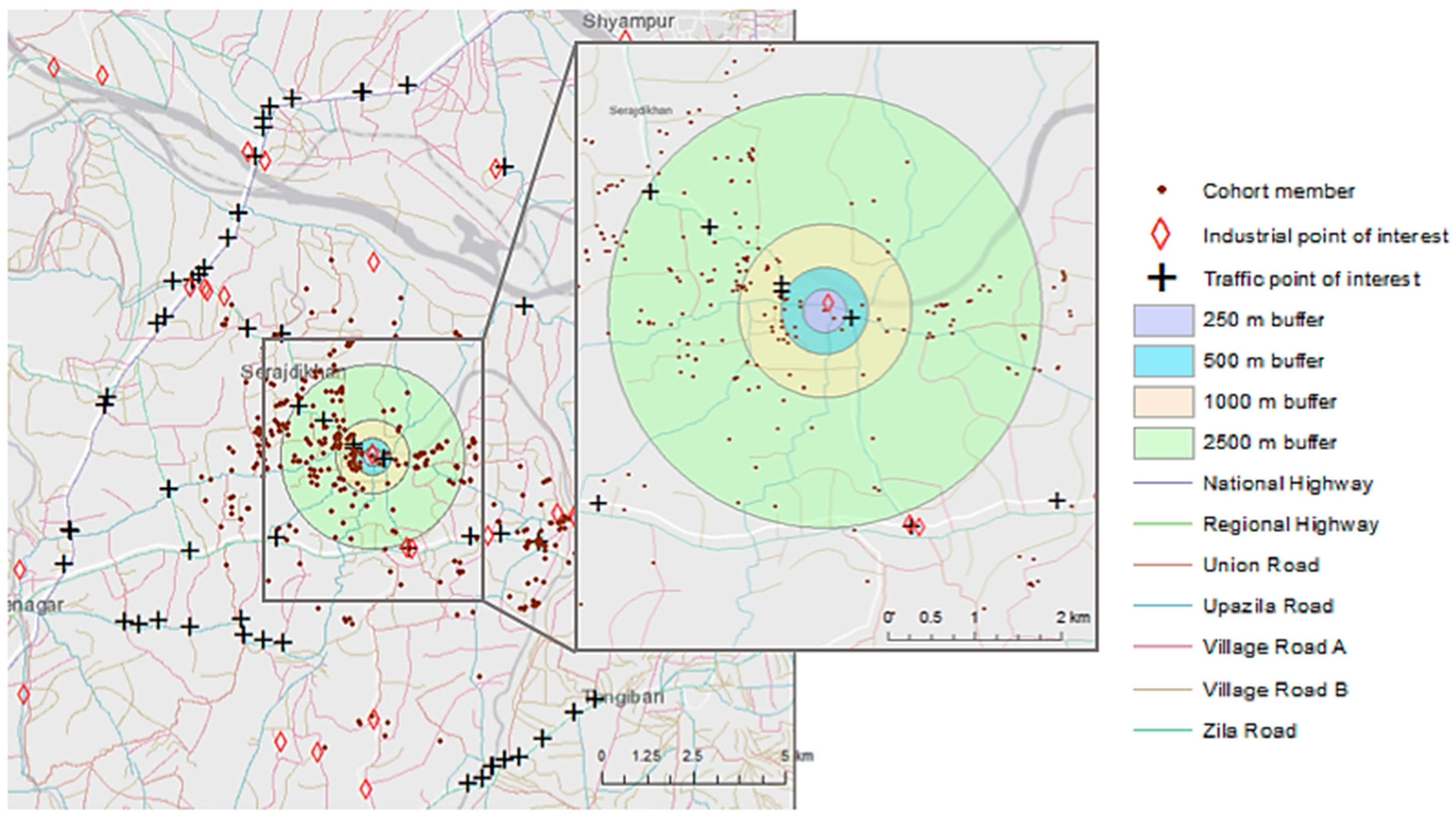
2.2.1. Sampling Site Selection
2.2.2. Air Sampling
2.2.3. Laboratory Analysis
2.3. Secondary Data Collection
2.4. Statistical Analyses—Ordinary Least Squares Regression
2.5. Statistical Analysis—Spatially Filtered Regression
β6(Maternal Educationfiltered) + β7(Maternal Educationspatial) + β8[Pbair, filtered] + β9[Pbair, spatial]
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Childhood Lead Poisoning; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Fischbein, A. Environmental and occupational lead exposure. In Environmental Occupational Medicine, 3rd ed.; Rom, W.N., Ed.; Lippincott-Raven: New York, NY, USA, 1998; pp. 969–994. [Google Scholar]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; Brubaker, C.J.; Adler, C.M.; Dietrich, K.N.; Altaye, M.A.; Egelhoff, J.C.; Wessel, S.; Elangovan, I.; Hornung, R.; Jarvis, K.; et al. Decreased Brain Volume in Adults with Childhood Lead Exposure. PLoS Med. 2008, 5, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E. Lead Exposure and Oxidative Stress-A Life Course Approach in US Adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Haque, A.; Islam, M.; Bashar, S.A. Lead poisoning: An alarming public health problem in Bangladesh. Int. J. Environ. Res. Public Health 2009, 6, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Ahua, E.; Saha, P.K. Prevalence of and risk factors for lead poisoning in young children in Bangladesh. J. Health Popul. Nutr. 2012, 30, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Henderson, A.K.; Daley, W.R.; Naughton, M.; Khan, M.H.; Rahman, M.; Kieszak, S.; Rubin, C.H. Blood lead levels of primary school children in Dhaka, Bangladesh. Environ. Health Perspect. 2001, 109, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Bellinger, D.C.; Valeri, L.; Hasan, M.O.; Quamruzzaman, Q.; Golam, M.; Kile, M.L.; Christiani, D.C.; Wright, R.O.; Mazumdar, M. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ. Health 2016, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Falk, H. International environmental health for the pediatrician: Case study of lead poisoning. Pediatrics 2003, 112 (Suppl. 1), 259–264. [Google Scholar] [PubMed]
- Bergkvist, C.; Kippler, M.; Hamadani, J.D.; Grander, M.; Tofail, F.; Berglund, M.; Vahter, M. Assessment of early-life lead exposure in rural Bangladesh. Environ. Res. 2010, 110, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Khan, M.H.; Khandker, S.; Sarwar, A.F.; Yasmin, N.; Faruquee, M.H.; Yasmin, R. Blood lead levels and health problems of lead acid battery workers in Bangladesh. Sci. World J. 2014, 2014, 974104. [Google Scholar] [CrossRef] [PubMed]
- Koplan, J.P.; Wells, A.V.; Diggory, H.J.; Baker, E.L.; Liddle, J. Lead Absorption in a community of potters in Barbados. Int. J. Epidemiol. 1977, 6, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Sharma, M.K. Blood lead level in the children of western Uttar Pradesh, India. Toxicol. Environ. Chem. 2011, 93, 504–512. [Google Scholar] [CrossRef]
- Meyer, P.A.; McGeehin, M.A.; Falk, H. A global approach to childhood lead poisoning prevention. Int. J. Hyg. Environ. Health 2003, 206, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). CDC’s Childhood Lead Poisoning Prevention Program: Blood Lead Levels in Children. Available online: https://www.cdc.gov/nceh/lead/about/program.htm (accessed on 30 July 2018).
- Centers for Disease Control and Prevention (CDC). Standard Surveillance Definitions and Classifications. Available online: https://www.cdc.gov/nceh/lead/data/definitions.htm (accessed on 30 July 2018).
- Gleason, K.; Shine, J.P.; Shobnam, N.; Rokoff, L.B.; Suchanda, H.S.; Ibne Hasan, M.O.; Mostofa, G.; Amarasiriwardena, C.; Quamruzzaman, Q.; Rahman, M.; et al. Contaminated turmeric is a potential source of lead exposure for children in rural Bangladesh. J. Environ. Public Health 2014, 2014, 730636. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, J.E.; Islam, M.S.; Parvez, S.M.; Raqib, R.; Rahman, M.S.; Muehe, E.M.; Luby, S.P. Prevalence of elevated blood lead levels among pregnant women and sources of lead exposure in rural Bangladesh: A case control study. Environ. Res. 2018, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Course WB2832: Lead Toxicity: What Are Routes of Exposure to Lead? ATSDR. Available online: https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=6 (accessed on 30 July 2018).
- Khaliquzzaman, M.; Biswas, S.K.; Tarafdar, S.A.; Islam, A.; Khan, A.H. Trace Element Composition of Airborne Particulate Matter in Urban and Rural Areas of Bangladesh; Bangladesh Atomic Energy Commission: Dhaka, Bangladesh, 1995; Volume 27. [Google Scholar]
- Boman, J.; Wagner, A.; Hossain, M.I. Elemental characterization of aerosols in urban and rural locations in Bangladesh. X-Ray Spectrom. 2005, 34, 460–467. [Google Scholar] [CrossRef]
- Begum, B.A.; Hopke, P.K.; Markwitz, A. Air pollution by fine particulate matter in Bangladesh. Atmos. Pollut. Res. 2013, 4, 75–86. [Google Scholar] [CrossRef]
- Ahmed, F.; Ishiga, H. Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmos. Environ. 2006, 40, 3835–3844. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). National Ambient Air Quality Standards (NAAQS) for Lead (Pb). Available online: https://www.epa.gov/lead-air-pollution/national-ambient-air-quality-standards-naaqs-lead-pb (accessed on 30 July 2018).
- Kile, M.L.; Baccarelli, A.; Tarantini, L.; Hoffman, E.; Wright, R.O.; Christiani, D.C. Correlation of global and gene-specific DNA methylation in maternal-infant pairs. PLoS ONE 2010, 5, e13730. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; Mazumdar, M.M.; Bobb, J.F.; Claus Henn, B.; Rodrigues, E.; Sharif, O.I.A.; Kile, M.L.; Quamruzzaman, Q.; Afroz, S.; Golam, M.; et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ. Health Perspect. 2017, 125, 067015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, S.M.; Talbott, E.O.; Brink, L.L.; Wu, C.; Sharma, R.K.; Marsh, G.M. Environmental lead and childhood blood lead levels in US children: NHANES, 1999–2006. Arch. Environ. Occup. Health 2017, 72, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Albalak, R.; Noonan, G.; Buchanan, S.; Flanders, W.D.; Gotway-Crawford, C.; Kim, D.; Jones, R.L.; Sulaiman, R.; Blumenthal, W.; Tan, R.; et al. Blood lead levels and risk factors for lead poisoning among children in Jakarta, Indonesia. Sci. Total Environ. 2003, 301, 75–85. [Google Scholar] [CrossRef]
- Dignam, T.; Rivera Garcia, B.; De Leon, M.; Curtis, G.; Creanga, A.A.; Azofeifa, A.; O’Neill, M.; Blanton, C.; Kennedy, C.; Rullan, M.; et al. Prevalence of Elevated Blood Lead Levels and Risk Factors Among Residents Younger Than 6 Years, Puerto Rico—2010. J. Public Health Manag. Pract. 2016, 22, E22–E35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Bretsch, J.K. Soil lead and children’s blood lead levels in Syracuse, NY, USA. Environ. Geochem. Health 2002, 24, 375–385. [Google Scholar] [CrossRef]
- Zahran, S.; Laidlaw, M.A.; McElmurry, S.P.; Filippelli, G.M.; Taylor, M. Linking source and effect: Resuspended soil lead, air lead, and children’s blood lead levels in Detroit, Michigan. Environ. Sci. Technol. 2013, 47, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- Environmental Systems Research Institute (ESRI). ArcGIS Desktop; 10.3; ESRI: Redlands, CA, USA, 2016. [Google Scholar]
- Open Data Kit Community. Open Data Kit (ODK); ODK Community, 2018. [Google Scholar]
- Kang, C.M.; Achilleos, S.; Lawrence, J.; Wolfson, J.M.; Koutrakis, P. Interlab comparison of elemental analysis for low ambient urban PM2.5 levels. Environ. Sci. Technol. 2014, 48, 12150–12156. [Google Scholar] [CrossRef] [PubMed]
- OpenStreetMap Contributors. Bangladesh; OpenStreetMap Foundation: Sutton Coldfield, UK, 2017. [Google Scholar]
- Google Developers. Google Maps API; Google, LLC: Mountain View, CA, USA, 2018. [Google Scholar]
- Morgenstern, V.; Zutavern, A.; Cyrys, J.; Brockow, I.; Gehring, U.; Koletzko, S.; Bauer, C.P.; Reinhardt, D.; Wichmann, H.E.; Heinrich, J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup. Environ. Med. 2007, 64, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, M.; Hoek, G.; van Vliet, P.; Meliefste, K.; Fischer, P.; Gehring, U.; Heinrich, J.; Cyrys, J.; Bellander, T.; Lewne, M.; et al. Estimating long-term average particulate air pollution concentrations: Application of traffic indicators and geographic information systems. Epidemiology 2003, 14, 228–239. [Google Scholar] [CrossRef] [PubMed]
- GIS Section. GIS Portal: A GIS Based Web Application for Project Planning of LGED; 2.0; Local Government Engineering Department (LGED): Dhaka, Bangladesh, 2017.
- Wong, D.W.; Yuan, L.; Perlin, S.A. Comparison of spatial interpolation methods for the estimation of air quality data. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thayn, J.B.; Simanis, J.M. Accounting for Spatial Autocorrelation in Linear Regression Models Using Spatial Filtering with Eigenvectors. Ann. Assoc. Am. Geogr. 2013, 103, 47–66. [Google Scholar] [CrossRef]
- Anselin, L. Spatial Econometrics: Methods and Models; Kluwer Academic: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Getis, A.; Griffith, D.A. Comparative Spatial Filtering in Regression Analysis. Geogr. Anal. 2002, 34, 130–140. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- World Health Organization. Exposure to Lead: A Major Public Health Concern; WHO Document Production Services: Geneva, Switzerland, 2010. [Google Scholar]
- Khan, A.H.; Khaliquzzaman, M.; Tarafdar, S.A.; Biswas, S.K.; Islam, A. Background Air Pollution Studies in Urban and Rural Areas of Bangladesh Using Nuclear-Related Analytical Techniques (NAHRES--19); International Atomic Energy Agency (IAEA): Dhaka, Bangladesh, 1994; pp. 47–57. [Google Scholar]
- International Agency for Research on Cancer. Air Pollution: Lead; WHO: Lyon, France, 2016. [Google Scholar]
- Bangladesh Bureau of Statistics (BBS); Statistics and Informatics Division (SID). District Statistics: Munshiganj; BBS: Dhaka, Bangladesh, 2011.
- Were, F.H.; Kamau, G.N.; Shiundu, P.M.; Wafula, G.A.; Moturi, C.M. Air and blood lead levels in lead acid battery recyling and manufacturing plants in Kenya. J. Occup. Environ. Hyg. 2012, 9, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, B.; Ravibabu, K.; Raghavan, S.; Krishnamurthy, V.; Rajan, B.K.; Rajmohan, H.R. Environmental and biological monitoring in a lead acid battery manufacturing unit in India. J. Occup. Health 2005, 47, 350–353. [Google Scholar] [CrossRef] [PubMed]
- McCann, M. Hazards in cottage industries in developing countries. Am. J. Ind. Med. 1996, 30, 125–129. [Google Scholar] [CrossRef]
- Matte, T.D.; Figueroa, J.P.; Ostrowski, S.; Burr, G.; Jackson-Hunt, L.; Baker, E.L. Lead exposure from conventional and cottage lead smelting in Jamaica. Arch. Environ. Contam. Toxicol. 1991, 21, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Koplan, J.P. Hazards of cottage and small industries in developing countries. Am. J. Ind. Med. 1996, 30, 123–124. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Course WB2832: Lead Toxicity: What Is the Biological Fate of Lead in the Body? ATSDR. Available online: https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=9 (accessed on 30 July 2018).
- World Bank. Bangladesh—Country Environmental Analysis (English); Paper No. 12; World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Kawai, M.; Toriumi, H.; Katagiri, Y.; Maruyama, Y. Home lead-work as a potential source of lead exposure for children. Int. Arch. Occup. Environ. Health 1983, 53, 37–46. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | No. (%) | Mean ± SD | |
|---|---|---|---|
| Age at follow-up | 22–26 months 27–31 months 32–36 months | 167 (43.4) 168 (43.6) 50 (12.9) | 28.23 ± 2.94 |
| Sex | Male Female | 202 (52.5) 183 (47.5) | |
| Maternal education | Primary or lower Secondary or more | 190 (49.4) 195 (50.6) | |
| Blood lead level (μg/dL) | 0–5 μg/dL 5–10 μg/dL 10–15 μg/dL 15–20 μg/dL >20 μg/dL | 52 (13.5) 199 (51.7) 101 (26.2) 24 (6.23) 9 (2.34) | 9.074 ± 4.50 |
| Sample and Site Type | Pb μg/m3 (SD) | TSP μg/m3 |
|---|---|---|
| Residential A | 2.04 (0.021) | 370.24 |
| Residential A | 1.37 (0.017) | 589.29 |
| Residential B | 0.14 (0.010) | 243.15 |
| Residential B | 0.21 (0.011) | 209.23 |
| Residential C | 3.10 (0.027) | 440.48 |
| Residential C | 2.50 (0.023) | 325.89 |
| Residential D | 0.19 (0.011) | 308.63 |
| Residential D | 0.20 (0.011) | 322.91 |
| Industrial, battery manufacturing | 376.58 (2.141) | 910.12 |
| Industrial, ceramics house | 0.47 (0.012) | 511.31 |
| Blank | 0.03 (0.009) | 2.083 |
| Blank | 0.02 (0.009) | 0.102 |
| Estimated Air Lead Concentration (μg/m3) | No. of Study Participants |
|---|---|
| <0.15 (NAAQS) | 0 |
| 0.16–0.5 | 104 |
| 0.6–1.0 | 120 |
| 1.1–1.5 | 101 |
| >1.5 | 60 |
| A. Blood Lead Levels and Estimated Air Lead | ||||||||
| Full Dataset (N = 385) | Excluding 2 Outliers (N = 383) | |||||||
| Coefficient | SE | t-Value | p-Value | Coefficient | SE | t-Value | p-Value | |
| Intercept | 10.39 | 2.31 | 4.49 | <0.001 | 9.94 | 2.04 | 4.86 | <0.001 |
| Estimated air Pb (μg/m3) | −0.40 | 0.38 | −1.04 | 0.30 | −0.31 | 0.34 | −0.91 | 0.36 |
| Age (months) | −0.04 | 0.08 | −0.45 | 0.66 | −0.04 | 0.07 | −0.53 | 0.59 |
| Sex (female) | 0.16 | 0.46 | 0.35 | 0.73 | −0.439 | 0.41 | 1.08 | 0.28 |
| Maternal education (primary or less) | 0.31 | 0.46 | −0.68 | 0.50 | 0.61 | 0.41 | −1.49 | 0.14 |
| Adjusted R2 = −0.0047 | Adjusted R2 = 0.0028 | |||||||
| B. Blood Lead Levels and Traffic and Proximity | ||||||||
| Full Dataset (N = 385) | Excluding 2 Outliers (N = 383) | |||||||
| Coefficient | SE | t-Value | p-Value | Coefficient | SE | t-Value | p-Value | |
| Intercept | 10.44 | 2.32 | 4.49 | <0.001 | 9.99 | 2.06 | 4.86 | <0.001 |
| No. of industrial sources within 1000 m | −0.01 | 0.32 | −0.02 | 0.98 | −0.14 | 0.31 | −0.46 | 0.64 |
| Length of major roads within 1000 m | −0.00008 | 0.0002 | −0.53 | 0.59 | 0.00002 | 0.0001 | 0.19 | 0.85 |
| Age (months) | −0.04 | 0.08 | −0.59 | 0.56 | −0.05 | 0.07 | −0.74 | 0.46 |
| Sex (female) | 0.21 | 0.47 | 0.44 | 0.66 | 0.48 | 0.41 | 1.16 | 0.25 |
| Maternal education (primary or less) | 0.32 | 0.47 | −0.75 | 0.45 | −0.61 | 0.41 | −1.48 | 0.14 |
| Adjusted R2 = −0.01 | Adjusted R2 = −0.001 | |||||||
| Covariate | Coefficient | SE | t-Value | p-Value | Moran’s I Z-Score |
|---|---|---|---|---|---|
| Intercept | 10.19 | 6.26 | 1.63 | 0.10 | |
| Air Pbfiltered | −3.41 | 4.14 | −0.83 | 0.41 | −0.104 |
| Agefiltered | 0.06 | 0.18 | 0.34 | 0.73 | 0.11 |
| Sexfiltered | 0.24 | 0.43 | 0.54 | 0.58 | −0.117 |
| Maternal educationfiltered | 1.83 | 2.04 | 0.90 | 0.36 | −0.05 |
| Air Pbspatial | −0.27 | 0.23 | −1.16 | 0.25 | 1.03 * |
| Agespatial | −0.07 | 0.07 | −0.93 | 0.35 | 0.82 * |
| Sexspatial | 1.93 | 1.08 | 1.78 | 0.08 | 0.416 * |
| Maternal educationspatial | 0.34 | 0.60 | 0.57 | 0.57 | −0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, M.K.; Young, E.S.; Mostofa, M.G.; Afroz, S.; Sharif Ibne Hasan, M.O.; Quamruzzaman, Q.; Bellinger, D.C.; Christiani, D.C.; Mazumdar, M. Lead in Air in Bangladesh: Exposure in a Rural Community with Elevated Blood Lead Concentrations among Young Children. Int. J. Environ. Res. Public Health 2018, 15, 1947. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15091947
Woo MK, Young ES, Mostofa MG, Afroz S, Sharif Ibne Hasan MO, Quamruzzaman Q, Bellinger DC, Christiani DC, Mazumdar M. Lead in Air in Bangladesh: Exposure in a Rural Community with Elevated Blood Lead Concentrations among Young Children. International Journal of Environmental Research and Public Health. 2018; 15(9):1947. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15091947
Chicago/Turabian StyleWoo, May K., Elisabeth S. Young, Md Golam Mostofa, Sakila Afroz, Md Omar Sharif Ibne Hasan, Quazi Quamruzzaman, David C. Bellinger, David C. Christiani, and Maitreyi Mazumdar. 2018. "Lead in Air in Bangladesh: Exposure in a Rural Community with Elevated Blood Lead Concentrations among Young Children" International Journal of Environmental Research and Public Health 15, no. 9: 1947. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15091947




