Removal Characteristics of N-Nitrosamines and Their Precursors by Pilot-Scale Integrated Membrane Systems for Water Reuse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling Protocol
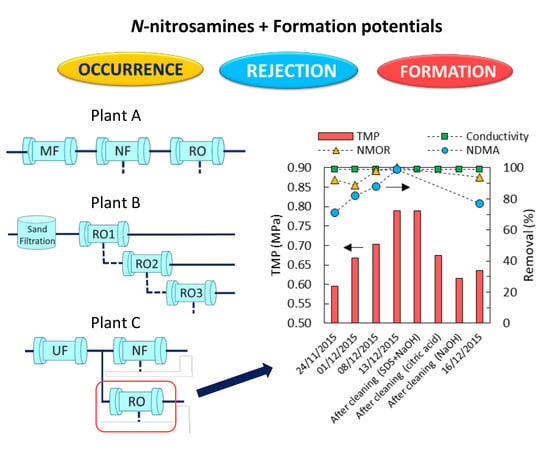
2.3. Chemical Cleaning of RO Element
2.4. Analytical Techniques
2.4.1. N-Nitrosamines
2.4.2. Formation Potentials
2.5. Calculations
3. Results and Discussion
3.1. Occurrence of N-Nitrosamines
3.2. Occurrence of Formation Potentials
3.3. Removal of N-Nitrosamines
3.3.1. Plant A
3.3.2. Plant B
3.3.3. Plant C
3.4. Impact of RO Membrane Fouling on N-Nitrosamines Removal
3.5. Rate of Reduction of N-Nitrosamine FPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of interest
References
- Drewes, J.E.; Amy, G.; Bellona, C.; Filteau, G. Comparing nanofiltration and reverse osmosis for treating recycled water. Water Environ. Res. Found. 2008, 100, 102–116. [Google Scholar]
- Alturki, A.A.; Tadkaew, N.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Memb. Sci. 2010, 365, 206–215. [Google Scholar] [CrossRef]
- Gerrity, D.; Pecson, B.; Trussell, R.S.; Trussell, R.R. Potable reuse treatment trains throughout the world. J. Water Supply Res. Technol. 2013, 62, 321–338. [Google Scholar] [CrossRef]
- Linge, K.L.; Blair, P.; Busetti, F.; Rodriguez, C.; Heitz, A. Chemicals in reverse osmosis-treated wastewater: Occurrence, health risk, and contribution to residual dissolved organic carbon. J. Water Supply Res. Technol. 2012, 61, 494–505. [Google Scholar] [CrossRef]
- Agus, E.; Zhang, L.; Sedlak, D.L. A framework for identifying characteristic odor compounds in municipal wastewater effluent. Water Res. 2012, 46, 5970–5980. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Plewa, M.J.; Mitch, W.A. N-Nitrosamines and halogenated disinfection byproducts in U.S. Full Advanced Treatment trains for potable reuse. Water Res. 2016, 101, 176–186. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Contaminant Candidate List 4—CCL4; Environmental Protection Agency: Washington, DC, USA, 2016.
- USEPA. Integrated Risk Information System (IRIS); Environmental Protection Agency: Washington, DC, USA, 1993.
- Krauss, M.; Longree, P.; Dorusch, F.; Ort, C.; Hollender, J. Occurrence and removal of N-Nitrosamines in wastewater treatment plants. Water Res. 2009, 43, 4381–4391. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Nakada, N.; Tanaka, H. Occurrence and fate of N-Nitrosamines and their formation potential in three wastewater treatment plants in Japan. Water Sci. Technol. 2013, 68, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, D.L.; Deeb, R.A.; Hawley, E.L.; Mitch, W.A.; Durbin, T.D.; Mowbray, S.; Carr, S. Sources and Fate of Nitrosodimethylamine and Its Precursors in Municipal Wastewater Treatment Plants. Water Environ. Res. 2005, 77, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, D.; Pisarenko, A.N.; Marti, E.; Trenholm, R.A.; Gerringer, F.; Reungoat, J.; Dickenson, E. Nitrosamines in pilot-scale and full-scale wastewater treatment plants with ozonation. Water Res. 2015, 72, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Roux, A.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-Nitrosamine rejection by reverse osmosis membranes: A full-scale study. Water Res. 2013, 47, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.J.; Döderer, K.; Keller, J.; Poussade, Y.; Mueller, J.; Gernjak, W. N-Nitrosodimethylamine (NDMA) in Purified Recycled Water Urban Water Security Research Alliance Technical Report No. 34; Urban Water Security Research Alliance: City Ease, Australia, 2010. [Google Scholar]
- Sgroi, M.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. N-Nitrosodimethylamine (NDMA) and its precursors in water and wastewater: A review on formation and removal. Chemosphere 2018, 191, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Mamo, J.; Insa, S.; Monclús, H.; Rodríguez-Roda, I.; Comas, J.; Barceló, D.; Farré, M.J. Fate of NDMA precursors through an MBR-NF pilot plant for urban wastewater reclamation and the effect of changing aeration conditions. Water Res. 2016, 102, 383–393. [Google Scholar] [CrossRef] [PubMed]
- NDMA and Other Nitrosamines Drinking Water Issues. Available online: https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/NDMA.html (accessed on 7 July 2018).
- Environment Protection and Heritage Council; National Health and Medical Research Council; Natural Resource Management Ministerial Council. Australian Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 2): Augmentation of Drinking Water Supplies. Available online: https://www.nhmrc.gov.au/guidelines-publications/eh56 (accessed on 7 July 2018).
- Miyashita, Y.; Park, S.-H.; Hyung, H.; Huang, C.-H.; Kim, J.-H. Removal of nitrosamines and their precursors by nanofiltration and reverse osmosis membranes. J. Environ. Eng. 2009, 135, 788–795. [Google Scholar] [CrossRef]
- Fujioka, T.; Nghiem, L.D.; Khan, S.J.; McDonald, J.A.; Poussade, Y.; Drewes, J.E. Effects of feed solution characteristics on the rejection of N-Nitrosamines by reverse osmosis membranes. J. Memb. Sci. 2012, 409, 66–74. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Roux, A.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-Nitrosamine rejection by nanofiltration and reverse osmosis membranes: The importance of membrane characteristics. Desalination 2013, 316, 67–75. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Henderson, R.K.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. Effects of membrane fouling on N-Nitrosamine rejection by nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2013, 427, 311–319. [Google Scholar] [CrossRef]
- Mitch, W.A.; Gerecke, A.C.; Sedlak, D.L. A N-Nitrosodimethylamine (NDMA) precursor analysis for chlorination of water and wastewater. Water Res. 2003, 37, 3733–3741. [Google Scholar] [CrossRef]
- Yoon, S.; Nakada, N.; Tanaka, H. Occurrence and removal of NDMA and NDMA formation potential in wastewater treatment plants. J. Hazard. Mater. 2011, 190, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Mccurry, D.L.; Ishida, K.P.; Oelker, G.L.; Mitch, W.A. Reverse osmosis shifts chloramine speciation causing re-formation of NDMA during potable reuse of wastewater. Environ. Sci. Technol. 2017, 41, 8589–8596. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Mitch, W.A.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Andrews, S.A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res. 2011, 45, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Young, T.M. NDMA formation during chlorination and chloramination of aqueous diuron solutions. Environ. Sci. Technol. 2008, 42, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Kohut, S.A.; Andrews, K.D. Polyelectrolyte age and N-Nitrosodimethylamine formation in drinking water treatment. Water Qual. Res. J. Can. 2003, 38, 719–735. [Google Scholar] [CrossRef]
- Kemper, J.M.; Walse, S.S.; Mitch, W.A. Quaternary amines as Nitrosamine precursors: A role for consumer products? Environ. Sci. Technol. 2010, 44, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Mitch, W.A. Contribution of N-Nitrosamines and their precursors to domestic sewage by greywaters and blackwaters. Environ. Sci. Technol. 2015, 49, 13158–13167. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Asami, M.; Konn, Y.; Oya, M.; Kunikane, S. Identification of antiyellowing agents as precursors of N-Nitrosodimethylamine production on ozonation from sewage treatment plant influent. Environ. Sci. Technol. 2009, 43, 5236–5241. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Asami, M.; Ohkubo, K.; Iwamoto, T.; Tanaka, Y.; Koshino, H.; Echigo, S.; Akiba, M. Identification of a new N-Nitrosodimethylamine precursor in sewage containing industrial effluents. Environ. Sci. Technol. 2014, 48, 11243–11250. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.J.; Döderer, K.; Hearn, L.; Poussade, Y.; Keller, J.; Gernjak, W. Understanding the operational parameters affecting NDMA formation at Advanced Water Treatment Plants. J. Hazard. Mater. 2011, 185, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Roccaro, P.; Oelker, G.L.; Snyder, S.A. N-Nitrosodimethylamine (NDMA) formation at an indirect potable reuse facility. Water Res. 2015, 70, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Khan, S.J.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-Nitrosamine removal by reverse osmosis for indirect potable water reuse—A critical review based on observations from laboratory-, pilot- and full-scale studies. Sep. Purif. Technol. 2012, 98, 503–515. [Google Scholar] [CrossRef]
- Mitch, W.A.; Sedlak, D.L. Characterization and fate of N-Nitrosodimethylamine precursors in municipal wastewater treatment plants. Environ. Sci. Technol. 2004, 38, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Plumlee, M.H.; López-Mesas, M.; Heidlberger, A.; Ishida, K.P.; Reinhard, M. N-Nitrosodimethylamine (NDMA) removal by reverse osmosis and UV treatment and analysis via LC-MS/MS. Water Res. 2008, 42, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.; Kim, S.H.; Cho, J. Removal of N-Nitrosamines in a membrane bioreactor and nanofiltration hybrid system for municipal wastewater reclamation: Process efficiency and mechanisms. Bioresour. Technol. 2015, 190, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Gohil, J.M.; Suresh, A.K. Chlorine attack on reverse osmosis membranes: Mechanisms and mitigation strategies. J. Memb. Sci. 2017, 541, 108–126. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Kim, T.U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Memb. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Altalyan, H.N.; Jones, B.; Bradd, J.; Nghiem, L.D.; Alyazichi, Y.M. Removal of volatile organic compounds (VOCs) from groundwater by reverse osmosis and nanofiltration. J. Water Process Eng. 2016, 9, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.R.; Agrawal, R.; Chellam, S. Temperature effects on sieving characteristics of thin-film composite nanofiltration membranes: Pore size distributions and transport parameters. J. Memb. Sci. 2003, 223, 69–87. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Ma, D.; Wang, Y.; Li, F.; Xu, X.; Xia, C.; Gao, B. Integration of coagulation and adsorption for removal of N-Nitrosodimethylamine (NDMA) precursors from biologically treated municipal wastewater. Environ. Sci. Pollut. Res. 2017, 24, 12426–12436. [Google Scholar] [CrossRef] [PubMed]








| Plant | Membrane | Membrane Material | Type | Pore Size a (µm) | NaCl Rejection a | Element Size | Number of Elements Per Vessel | Number of Vessels Per Unit | Recovery Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| A | MF | Ceramic | Cylindrical | 0.1 | N.A. | N.A. | N.A. | N.A. | 100 |
| NF | Poly-vinyl alcohol polyamide | Spiral | N.A. | 92% | 4 inch | 1 | 1 | 65 | |
| RO | Fully aromatic polyamide | Spiral | N.A. | 99.5% | 4 inch | 1 | 1 | 80 | |
| B | RO | Aromatic composite polyamide | Spiral | N.A. | 99.7% | 4 inch | 1 | 7 | 67 |
| C | UF | Poly-vinylidene fluoride | Hollow fiber | 0.01 | N.A. | N.A. | N.A. | N.A. | 100 |
| NF | Piperazine polyamide | Spiral | N.A. | 60% | 4 inch | 1 | 1 | 75 | |
| RO | Aromatic composite polyamide | Spiral | N.A. | 99.7% | 4 inch | 1 | 1 | 75 |
| Compound | NDMA | NMEA | NPYR | NDEA | NPIP | NMOR | NDPA | NDBA |
|---|---|---|---|---|---|---|---|---|
| Molecular weight (g/mol) a | 74.08 | 88.11 | 100.12 | 102.14 | 114.15 | 116.12 | 130.19 | 158.25 |
| a | 3.22 | 3.42 | 3.30 | 3.32 | 3.30 | 3.14 | 3.30 | 3.30 |
| a | 0.08 | 0.41 | 0.39 | 0.75 | 0.81 | −0.32 | 1.05 | 2.56 |
| Sampling Campaign | Plant A | Plant B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | Concentration (mg/L) | ||||||||||
| NF Perm. | NF Conc. | RO Perm. | RO Conc. | Sand Filter Eff. | 1st RO Perm. | 1st RO Conc. | 2nd RO Perm. | 2nd RO Conc. | 3rd RO Perm. | 3rd RO Conc. | |
| 1st | 0.33 | 0.29 | 0.20 | 0.31 | 1.03 | 0.41 | 0.65 | 0.30 | 0.81 | 0.38 | 0.81 |
| 2nd | 1.18 | 1.15 | 1.18 | 1.20 | 0.98 | 0.19 | 0.35 | <0.02 | 0.40 | <0.02 | 0.46 |
| 3rd | 0.03 | 0.09 | <0.02 | 0.02 | 3.24 | 0.42 | 0.67 | 0.21 | 0.39 | 0.13 | 0.33 |
| Plant | Sampling Campaign | Membrane Process | Permeate Flux (L/m² h) | TMP (MPa) a | Feed Temperature (°C) | Feed pH | Feed Conductivity (µS/cm) |
|---|---|---|---|---|---|---|---|
| A | 1st | NF | 24.8 | 0.41 | 16.1 | 8.1 | 815 |
| RO | 24.8 | 0.48 | 16.2 | 7.9 | 579 | ||
| 2nd | NF | 24.8 | 0.38 | 16.5 | 7.8 | 765 | |
| RO | 24.8 | 0.48 | 16.7 | 7.1 | 515 | ||
| 3rd | NF | 24.8 | 0.34 | 29.2 | 7.0 | 867 | |
| RO | 24.8 | 0.44 | 29.5 | 7.1 | 577 | ||
| B | 1st | 1st-stage RO | N.A. | N.A. | 18.3 | 5.3 | 582 |
| 2nd-stage RO | N.A. | N.A. | 18.9 | 5.9 | 739 | ||
| 3rd-stage RO | N.A. | N.A. | 19.3 | 5.2 | 880 | ||
| 2nd | 1st-stage RO | N.A. | N.A. | 25.3 | 6.5 | 1230 | |
| 2nd-stage RO | N.A. | N.A. | 25.7 | 5.1 | 1290 | ||
| 3rd-stage RO | N.A. | N.A. | 26.0 | 6.5 | 1830 | ||
| 3rd | 1st-stage RO | N.A. | N.A. | 25.3 | 5.9 | 739 | |
| 2nd-stage RO | N.A. | N.A. | 25.7 | 6.5 | 1230 | ||
| 3rd-stage RO | N.A. | N.A. | 26.0 | 6.5 | 1830 | ||
| C | 1st | RO | 11.3 | 0.54 | 30.9 | 7.4 | 1830 |
| 2nd | RO | 11.3 | 0.57 | 31.7 | N.A. | 1377 | |
| 3rd | RO | 11.3 | 0.58 | 32.6 | N.A. | 1465 | |
| 4th | RO | 12.8 | 0.74 | 30.8 | 7.4 | 2520 | |
| 5th | RO | 11.3 | 0.71 | 24.7 | 7.4 | 1570 |
| Sampling Campaign | Membrane Process | Water Recovery (%) | Permeate Flux (L/m²h) | TMP (MPa) a | Feed Temperature (°C) | Feed Conductivity (µS/cm) | Feed TOC (mg/L) |
|---|---|---|---|---|---|---|---|
| 1st | RO | 57 | 21.5 | 0.60 | 29.9 | 1600 | 13.9 |
| 2nd | RO | 56 | 21.0 | 0.67 | 28.4 | 1560 | 16.0 |
| 3rd | RO | 55 | 20.7 | 0.70 | 27.4 | 1670 | 18.0 |
| 4th | RO | 57 | 21.2 | 0.79 | 27.3 | 1650 | 14.6 |
| 5th | RO | 56 | 21.1 | 0.64 | 26.6 | 1730 | 14.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, H.; Yamashita, N.; Nakada, N.; Tanaka, H. Removal Characteristics of N-Nitrosamines and Their Precursors by Pilot-Scale Integrated Membrane Systems for Water Reuse. Int. J. Environ. Res. Public Health 2018, 15, 1960. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15091960
Takeuchi H, Yamashita N, Nakada N, Tanaka H. Removal Characteristics of N-Nitrosamines and Their Precursors by Pilot-Scale Integrated Membrane Systems for Water Reuse. International Journal of Environmental Research and Public Health. 2018; 15(9):1960. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15091960
Chicago/Turabian StyleTakeuchi, Haruka, Naoyuki Yamashita, Norihide Nakada, and Hiroaki Tanaka. 2018. "Removal Characteristics of N-Nitrosamines and Their Precursors by Pilot-Scale Integrated Membrane Systems for Water Reuse" International Journal of Environmental Research and Public Health 15, no. 9: 1960. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15091960