Effectiveness and Estimation of Cost-Effectiveness of a Group-Based Multicomponent Physical Exercise Programme on Risk of Falling and Frailty in Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants and Inclusion Criteria
2.3. Outcome Measures
2.3.1. Primary Outcomes
2.3.2. Secondary Outcomes
2.4. Intervention
2.5. Data Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ong, A.D.; Uchino, B.N.; Wethington, E. Loneliness and Health in Older Adults: A Mini-Review and Synthesis. Gerontology 2016, 62, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.M.; Lamb, S.E. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 9, CD007146. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Injury Database. European Commission, 2016. Available online: http://ec.europa.eu/health/data_collection/databases/idb/index_en.htm (accessed on 2 May 2018).
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Toward a conceptual definition of frail community dwelling older people. Nurs. Outlook 2010, 58, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.; van Assen, M.A.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. The Tilburg Frailty Indicator: Psychometric properties. J. Am. Med. Dir. Assoc. 2010, 11, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Markle-Reid, M.; Browne, G. Conceptualizations of frailty in relation to older adults. J. Adv. Nurs. 2003, 44, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Paul, C.; Gobbens, R.; Fernandes, L. Frailty as a predictor of short-term adverse outcomes. PeerJ 2015, 3, 1121. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Romero, L.; Abizanda, P. Fragilidad como predictor de episodios adversos en estudios epidemiológicos: Revisión de la literatura. Rev. Esp. Geriatr. Gerontol. 2013, 48, 285–289. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2007; Available online: http://www.who.int/entity/ageing/publications/Falls_prevention7March.pdf (accessed on 2 May 2018).
- World Health Organization. WHO Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2012; Available online: http://www.who.int/ageing/publications/Falls_prevention7March.pdf?ua=1 (accessed on 2 May 2018).
- Bock, J.O.; König, H.H.; Brenner, H.; Haefeli, W.E.; Quinzler, R.; Matschinger, H.; Saum, K.U.; Schöttker, B.; Heider, D. Associations of frailty with health care costs—Results of the ESTHER cohort study. BMC Health Serv. Res. 2016, 16, 128. [Google Scholar] [CrossRef]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, R.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas Herrero, A.; Izquierdo, M. Physical exercise as an efficient intervention in frail elderly persons. An. Sist. Sanit. Navar. 2012, 35, 69–85. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, F.; Cassou, B.; Charles, M.A.; Dargent-Molina, P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f6234. [Google Scholar] [PubMed]
- Sherrington, C.; Whitney, J.C.; Lord, S.R.; Herbert, R.D.; Cumming, R.G.; Close, J.C. Effective exercise for the prevention of falls: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2008, 56, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. N. S. W. Public Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef]
- Campbell, A.J.; Robertson, M.C. The Otago Exercise Programme to Prevent Falls in Older: A Homebased; Individually Tailored Strength and Balance Retraining Programme; Otago Medical School, University of Otago: Dunedin, New Zealand, 2003. [Google Scholar]
- Stevens, J.A.; Burns, E. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults; National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015.
- Thomas, S.; Mackintosh, S.; Halbert, J. Does the ‘Otago exercise programmeme’ reduce mortality and falls in older adults?: A systematic review and metaanalysis. Age Ageing 2010, 39, 681–687. [Google Scholar] [CrossRef]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; D’Avanzo, B.; Gwyther, H.; et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 140–232. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Moen, K.; Roysland, A.S.; Helbostad, J.L. The Otago exercise programme performed as group training versus home training in fall-prone older people: A randomized controlled trial. Physiother. Res. Int. 2014, 92, 108–116. [Google Scholar] [CrossRef]
- American Geriatrics Society, British Geriatrics Society. AGS/BGS Clinical Practice Guideline: Prevention of Falls in Older Persons; American Geriatrics Society: New York, NY, USA, 2010. [Google Scholar]
- Curcio, C.L.; Gómez, F. Temor a caer en ancianos: Controversias en torno a un concepto y a su medición. Hacia la Promoción de la Salud 2012, 17, 186–204. [Google Scholar]
- Ambrose, A.F.; Paul, G.; Hausdorffb, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef]
- NICE. Falls: Assessment and Prevention of Falls in Older People; Clinical Guideline; No. 161; National Institute for Health and Care Excellence (NICE): London, UK, 2013. [Google Scholar]
- Gobbens, R.J.; Schols, J.M.; van Assen, M.A. Exploring the efficiency of the Tilburg Frailty Indicator: A review. Clin. Interv. Aging 2017, 12, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Suurmeijer, T.P.B.M.; Kempen, G.I.J.M. Behavioural changes as an outcome of disease: The development of an instrument. Int. J. Health Sci. 1990, 1, 189–194. [Google Scholar]
- Orem, D.E. Nursing: Concepts of Practice, 5th ed.; Mosby: Saint Louis, MO, USA, 1995. [Google Scholar]
- Alhambra-Borrás, T.; Durá-Ferrandis, E.; Garcés-Ferrer, J.; Sánchez-García, J. The Appraisal of Self-care Agency scale Revised (ASAR): Adaptation and validation in a Spanish seniors’ sample. Span. J. Psychol. 2017, 20, e48. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinetti, M.E.; Richman, D.; Powell, L. Falls efficacy as a measure of fear of falling. J. Gerontol. 1990, 45, 239–243. [Google Scholar] [CrossRef]
- Litwin, H.; Erlich, B.; Dunsky, A. The Complex Association between Fear of Falling and Mobility Limitation in Relation to Late-Life Falls: A SHARE-Based Analysis. J. Aging Health 2018, 30, 987–1008. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinsiki, M.; Keller, S.D. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, P.N.; Shumway-Cook, A.; Ciol, M.A. The effects of a home-based exercise programme on physical function in frail older adults. J. Geriatr. Phys. Ther. 2010, 33, 78–84. [Google Scholar] [PubMed]
- Boehler, C.E.; de Graaf, G.; Steuten, L.; Yang, Y.; Abadie, F. Development of a Web-based Tool for the Assessment of Health and Economic Outcomes of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA). BMC Med. Inform. Decis. Mak. 2015, 3, S4. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Rivero-Arias, O.; Clarke, P. Estimating the association between SF-12 responses and EQ-5D utility values by response mapping. Med. Decis. Mak. 2006, 26, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.; van Rossum, E.; de Witte, L.; Kempen, G.I.; van den Heuvel, W. Interventions to prevent disability in frail community-dwelling elderly: A systematic review. BMC Health Serv. Res. 2008, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.M.; van Ravensbergb, C.D.; Hobbelenb, J.S.M.; Olde Rikkertd, M.G.M.; Staal, J.B.; Nijhuis-van der Sandena, M.W.G. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: A meta-analysis. Ageing Res. Rev. 2012, 11, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Giné-Garriga, M.; Roqué-Fíguls, M.; Coll-Planas, L.; Sitja-Rabert, M.; Salva, A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Luger, E.; Kapan, A.; Titze, S.; Lackinger, C.; Schindler, K.E.; Dorner, T.E. Associations between daily physical activity, handgrip strength, muscle mass, physical performance and quality of life in prefrail and frail community-dwelling older adults. Qual. Life Res. 2016, 25, 3129–3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The effectiveness of exercise interventions for the management of frailty: A systematic review. J. Aging Res. 2011, 2011, 569194. [Google Scholar] [CrossRef] [PubMed]
- Abizanda, P.; Gómez-Pavón, J.; Martín-Lesende, I.; Baztán, J.J. Detección y prevención de la fragilidad: Una nueva perspectiva de prevención de la dependencia en las personas mayores. Med. Clín. 2010, 135, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Funes, J.A.; Helmer, C.; Amieva, H.; Barberger-Gateau, P.; Le Goff, M.; Ritchie, K.; Portet, F.; Carrière, I.; Tavernier, B.; Gutiérrez-Robledo, L.M.; et al. Frailty among community-dwelling elderly people in France: The three-city study. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Hwang, C.L.; Wu, Y.T. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Arch. Phys. Med. Rehabil. 2012, 93, 237–244. [Google Scholar] [CrossRef]
- Eckman, M. Enfermería Geriátrica; Manual Moderno: Mexico DF, Mexico, 2012. [Google Scholar]
- Kojima, G. Frailty as a predictor of hospitalisation among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.C.; Devlin, N.; Gardner, M.M.; Campbell, A.J. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. BMJ 2001, 322, 697. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Robertson, M.C.; Ashe, M.C.; Liu-Ambrose, T.; Khan, K.M.; Marra, C.A. Does a home based strength and balance programme in people aged ≥ 80 years provide the best value for money to prevent falls? A systematic review of economic analyses of falls prevention interventions. Br. J. Sports Med. 2010, 44, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Balzer, K.; Bremer, M.; Schramm, S.; Lühmann, D.; Raspe, H. Falls prevention for the elderly. GMS Health Technol. Assess. 2012, 8. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Tathan, R.L.; Black, W.C. Multivariate Data Analysis, 5th ed.; Pearson: London, UK, 1999. [Google Scholar]

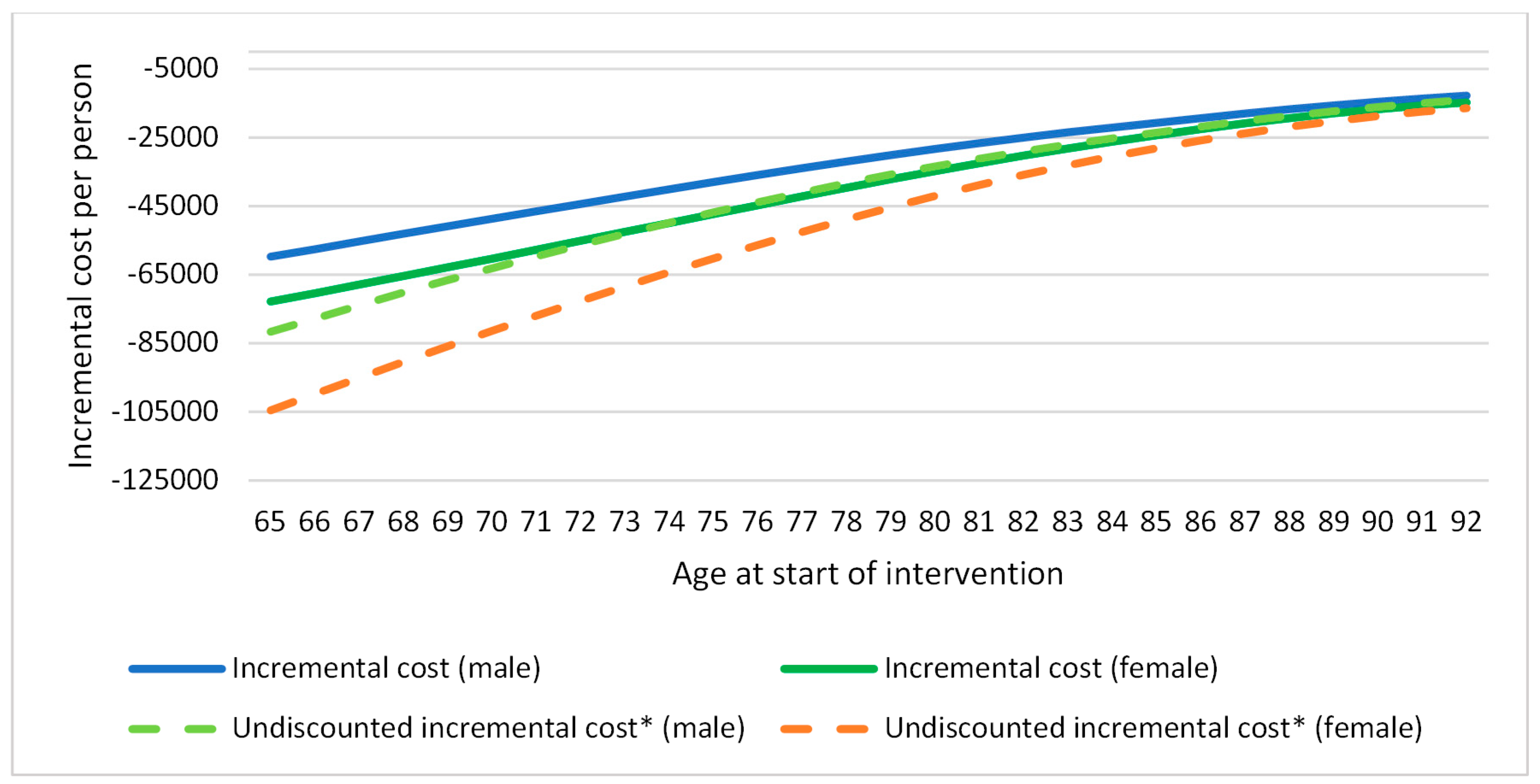
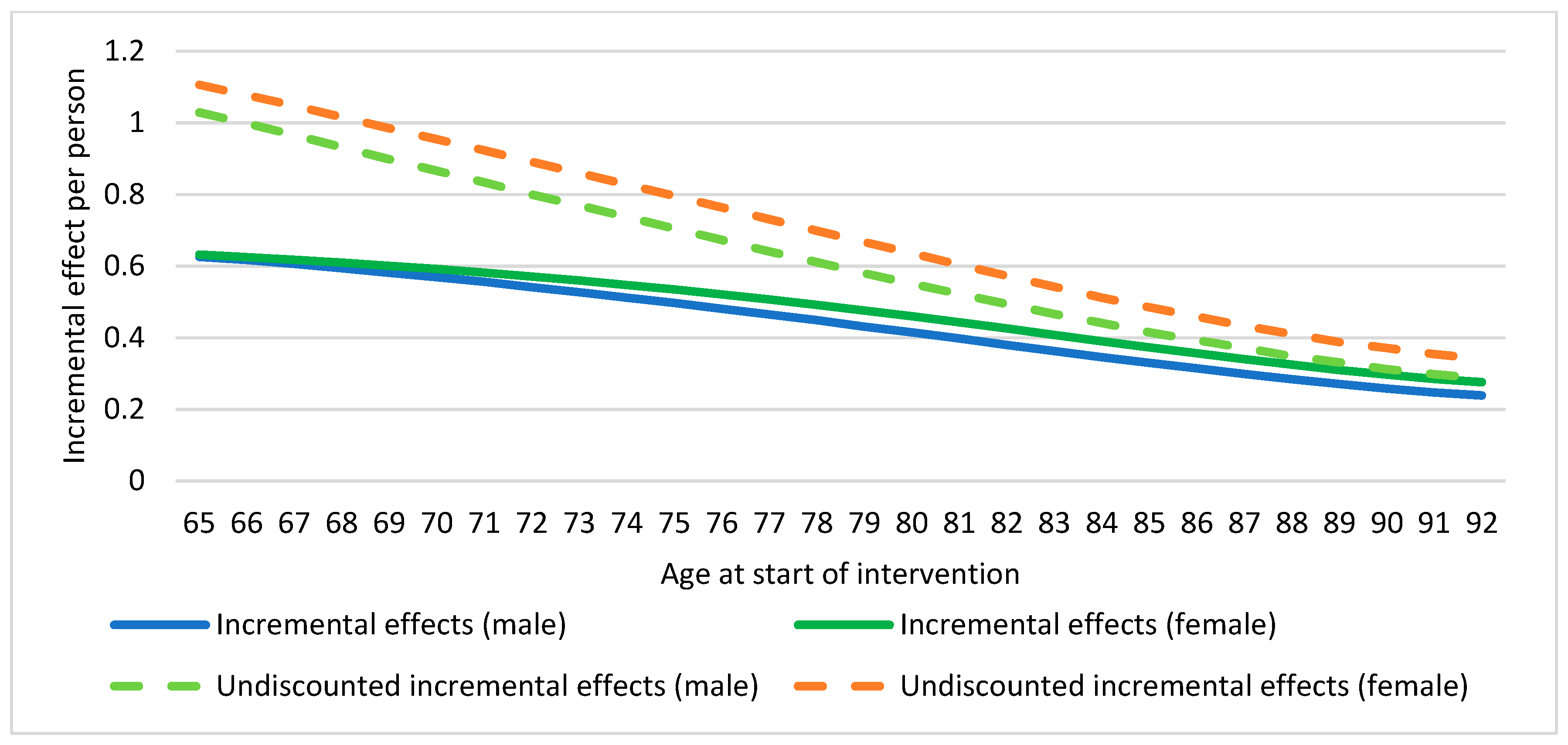
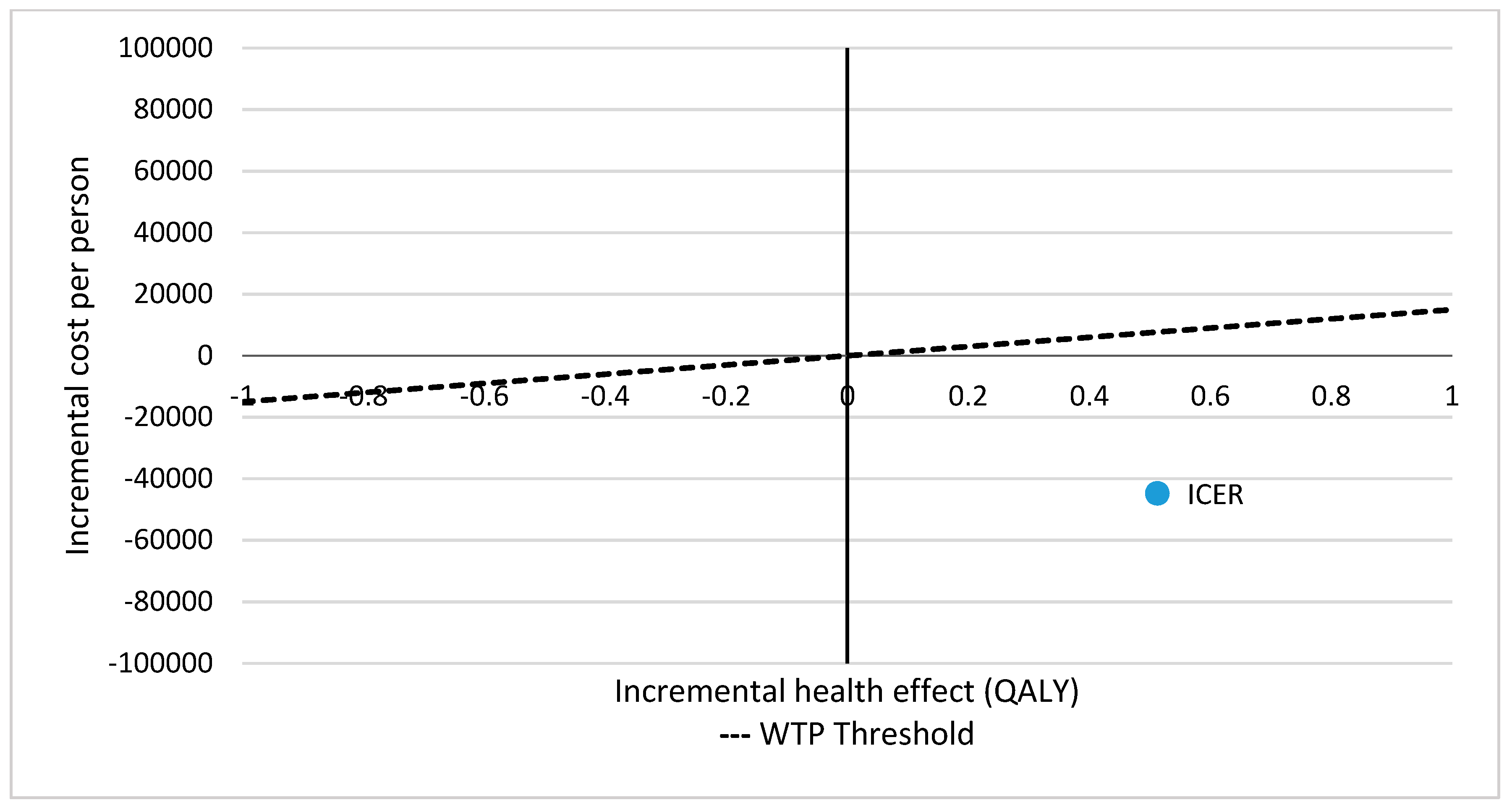
| Risk of Falling Differences | Intervention Group | Comparison Group | Intergroup Differences |
|---|---|---|---|
| PRE n (%) | 50 (90.9%) | 86 (63.2%) | X2 = 14.628; p = 0.000 |
| POST n (%) | 25 (45.4%) | 87 (64.0%) | X2 = 5.536; p = 0.019 |
| % of change | −45.5% | +0.8% | |
| Intragroup differences | McNemar; p = 0.000 | McNemar; p = 1.000 | |
| Effect size ** | 0.29 | 0.38 |
| Frailty Differences | Intervention Group | Comparison Group | Intergroup Differences |
|---|---|---|---|
| PRE Mean ± SD | 6.20 ± 3.15 | 5.21 ± 2.24 | U de Mann–Whitney = 3031.5; p = 0.039 |
| POST Mean ± SD | 4.27 ± 2.69 | 5.01 ± 2.26 | U de Mann–Whitney = 3054.5; p = 0.046 |
| % of change | −31% | −4% | |
| Intragroup differences | Wilcoxon z = −4.373; p = 0.000 | Wilcoxon z = −1.142; p = 0.253 | |
| Effect size ** | 0.59 | 0.09 |
| Physical Performance and Body Composition Variables | PRE Mean ± SD | POST Mean ± SD | Pre-Post Difference | Effect Size ** |
|---|---|---|---|---|
| Physical Performance (SPPB) | ||||
| SPPB total | 7.71 ± 2.07 | 8.35 ± 2.15 | p = 0.009 | 0.35 |
| Balance test | 3.18 ± 0.98 | 3.51 ± 0.84 | p = 0.009 | 0.35 |
| Gait speed test | 2.44 ± 1.03 | 2.62 ± 1.01 | p = 0.176 | 0.18 |
| Chair stand test | 2.09 ± 1.06 | 2.22 ± 1.17 | p = 0.225 | 0.16 |
| Body composition (Tanita scale) | ||||
| Weight | 70.95 ± 14.02 | 70.39 ± 13.9 | p = 0.152 | 0.07 |
| BMI | 30.96 ± 6.07 | 30.55 ± 5.95 | p = 0.045 | 0.07 |
| Body fat (kg) | 27.61 ± 10.07 | 26.95 ± 9.87 | p = 0.075 | 0.04 |
| Lean body mass (kg) | 43.34 ± 6.66 | 42.72 ± 8.52 | p = 0.930 | 0.01 |
| Body water (kg) | 31.95 ± 4.78 | 31.82 ± 4.95 | p = 0.868 | 0.02 |
| Health-Related Variables | Intervention Group | Comparison Group | |
|---|---|---|---|
| SF-12 Physical Health | % of change | +7% | −9% |
| Intragroup difference | p = 0.200 | p = 0.001 | |
| Effect size ** | 0.17 | 0.28 | |
| SF-12 Mental Health | % of change | −2% | −7% |
| intragroup difference | p = 0.535 | p = 0.003 | |
| Effect size ** | 0.08 | 0.25 | |
| Self-care agency | % of change | +7% | +8% |
| Intragroup difference | p = 0.001 | p = 0.000 | |
| Effect size ** | 0.48 | 0.42 | |
| Limitation in ADLs | % of change | −6% | +3% |
| Intragroup difference | p = 0.011 | p = 0.905 | |
| Effect size ** | 0.35 | 0.01 | |
| Falls self-efficacy | % of change | −9% | +4% |
| Intragroup difference | p = 0.098 | p = 0.298 | |
| Effect size ** | 0.22 | 0.09 | |
| Average doctor visits | % of change | −33% | −7% |
| Intragroup difference | p = 0.000 | p = 0.070 | |
| Effect size ** | 0.48 | 0.15 | |
| Hospitalisation | % of change | −50% | +14% |
| Intragroup difference | p = 0.508 | p = 0.832 | |
| Effect size ** | 0.08 | 0.18 | |
| Variables Used in MAFEIP | Intervention Group | Comparison Group |
|---|---|---|
| Costs | ||
| Recurring cost per patient/year (intervention) Healthcare cost—baseline Healthcare cost—deteriorated | 104€ 1615.02€ 3130.96€ | - 1630.22€ 9030.13€ |
| Utility ** | ||
| Baseline state * Deteriorated state * | 0.81 0.75 | 0.81 0.75 |
| Incremental Cost and HRQoL * Effects | Effect Result |
|---|---|
| Incremental cost (healthcare) | −44,832.92 |
| Incremental effects | 0.513 |
| Incremental cost-effectiveness ratio (healthcare) | Dominant |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhambra-Borrás, T.; Durá-Ferrandis, E.; Ferrando-García, M. Effectiveness and Estimation of Cost-Effectiveness of a Group-Based Multicomponent Physical Exercise Programme on Risk of Falling and Frailty in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2019, 16, 2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16122086
Alhambra-Borrás T, Durá-Ferrandis E, Ferrando-García M. Effectiveness and Estimation of Cost-Effectiveness of a Group-Based Multicomponent Physical Exercise Programme on Risk of Falling and Frailty in Community-Dwelling Older Adults. International Journal of Environmental Research and Public Health. 2019; 16(12):2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16122086
Chicago/Turabian StyleAlhambra-Borrás, Tamara, Estrella Durá-Ferrandis, and Maite Ferrando-García. 2019. "Effectiveness and Estimation of Cost-Effectiveness of a Group-Based Multicomponent Physical Exercise Programme on Risk of Falling and Frailty in Community-Dwelling Older Adults" International Journal of Environmental Research and Public Health 16, no. 12: 2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16122086




