Sedentary Behavior: A Key Component in the Interaction between an Integrated Lifestyle Approach and Cardiac Autonomic Function in Active Young Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Experimental Design
2.3. Instruments
2.3.1. Physical Assessment
2.3.2. Physical Activity and Sedentary Behavior
2.3.3. Sleep Quality
2.3.4. Heart Rate Variability Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Hemodynamic Variables and Heart Rate Variability in the Different Positions
4.2. Unadjusted Model and Heart Rate Variability
4.3. Adjusted Model and Heart Rate Variability
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chastin, S.F.M.; Ferriolli, E.; Stephens, N.A.; Fearon, K.C.; Greig, C. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing 2012, 41, 111–114. [Google Scholar] [CrossRef]
- Stewart, K.J. Physical activity and aging. Ann. N. Y. Acad. Sci. 2005, 1055, 193–206. [Google Scholar] [CrossRef]
- Netzer, N.; Strohl, K.P. Sleep and Aging. In Competencies in Sleep Medicine; Springer: New York, NY, USA, 2014; pp. 325–342. [Google Scholar]
- Powers, S. Exercise Physiology: Theory and Application to Fitness and Performance; McGraw-Hill Higher Education: New York, NY, USA, 2014. [Google Scholar]
- Task Force of the European Society of Cardiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Pradhapan, P.; Tarvainen, M.P.; Nieminen, T.; Lehtinen, R.; Nikus, K.; Kähönen, M.; Viik, J.; Lehtimäki, T.; Kähönen, M. Effect of heart rate correction on pre- and post-exercise heart rate variability to predict risk of mortality—An experimental study on the FINCAVAS cohort. Front. Physiol. 2014, 5, 208. [Google Scholar] [CrossRef]
- Melanson, E.L. Resting heart rate variability in men varying in habitual physical activity. Med. Sci. Sports Exerc. 2000, 32, 1894–1901. [Google Scholar] [CrossRef]
- Hallman, D.M.; Sato, T.; Kristiansen, J.; Gupta, N.; Skotte, J.; Holtermann, A.; Tchounwou, P.B. Prolonged Sitting is Associated with Attenuated Heart Rate Variability during Sleep in Blue-Collar Workers. Int. J. Environ. Public Health 2015, 12, 14811–14827. [Google Scholar] [CrossRef] [Green Version]
- Fenton, S.A.M.; Van Zanten, J.J.C.S.V.; Kitas, G.D.; Duda, J.L.; Rouse, P.C.; Yu, C.-A.; Metsios, G.S. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet. Disord. 2017, 18, 282. [Google Scholar] [CrossRef]
- Tobaldini, E.; Nobili, L.; Strada, S.; Casali, K.R.; Braghiroli, A.; Montano, N. Heart rate variability in normal and pathological sleep. Front. Physiol. 2013, 4, 294. [Google Scholar] [CrossRef] [Green Version]
- Burton, A.R.; Rahman, K.; Kadota, Y.; Lloyd, A.; Vollmer-Conna, U. Reduced heart rate variability predicts poor sleep quality in a case–control study of chronic fatigue syndrome. Exp. Brain Res. 2010, 204, 71–78. [Google Scholar] [CrossRef]
- Hall, M.; Vasko, R.; Buysse, D.; Ombao, H.; Chen, Q.; Cashmere, J.D.; Kupfer, D.; Thayer, J.F. Acute Stress Affects Heart Rate Variability During Sleep. Psychosom. Med. 2004, 66, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Caserotti, P.; Patel, K.V.; Matthews, C.E.; Berrigan, D.; Van Domelen, D.R.; Brychta, R.J.; Chen, K.Y.; Harris, T.B. Association of Sedentary Time with Mortality Independent of Moderate to Vigorous Physical Activity. PloS ONE 2012, 7, e37696. [Google Scholar] [CrossRef]
- Brown, W.J.; Bauman, A.E.; Bull, F.C.; Burton, N.W. Development of Evidence-Based Physical Activity Recommendations for Adults (18–64 years): Report Prepared for the Australian Government Department of Health, August 2012; Commonwealth of Australia: Canberra, Australia, 2013.
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise; American College of Sports Medicine: Indianapolis, IN, USA, 2011. [Google Scholar]
- Janssen, I.; Hicks, A.; Murumets, K.; Duggan, M.; Tremblay, M.S.; Leblanc, A.G.; Kho, M.E.; Colley, R.C. Canadian Sedentary Behaviour Guidelines for Children and Youth. Appl. Physiol. Nutr. Metab. 2011, 36, 59–64. [Google Scholar] [Green Version]
- Balady, G.J.; Berra, K.A.; Golding, L.A.; Gordon, N.F.; Mahler, D.A.; Myers, J.N.; Sheldahl, L.M. Diretrizes do ACSM para os Testes de Esforço e sua Prescrição; Guanabara Koogan: Rio de Janeiro, Brazil, 2003; 239p. [Google Scholar]
- Ministério da Saúde. Resolução nº. 196 de 10 de outubro de 1996. Diretrizes e Normas Regulamentadoras de Pesquisas Envolvendo Seres Humanos. Diário Of. Da União 1996, 4 (Suppl. S2). [Google Scholar]
- Porto, L.G.G.; Junqueira, L.F., Jr. Comparison of time-domain short-term heart interval variability analysis using a wrist-worn heart rate monitor and the conventional electrocardiogram. Pacing Clin. Electrophysiol. 2009, 32, 43–51. [Google Scholar] [CrossRef]
- Molina, G.E.; Fontana, K.E.; Porto, L.G.G.; Junqueira, J.L.F. Post-exercise heart-rate recovery correlates to resting heart-rate variability in healthy men. Clin. Auton. 2016, 26, 415–421. [Google Scholar] [CrossRef]
- Nogueira, A.D.R.; Muxfeldt, E.; Salles, G.F.; Bloch, K.V. A importância clínica da pressão de pulso. Rev. Bras. Hipertens. 2003, 10, 10–12. [Google Scholar]
- Pardini, R.; Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, E.; Braggion, G.; Andrade, D.; Oliveira, L.; Figueira, A.; Raso, V. Validação do questionário internacional de nível de atividade física (IPAQ-versão 6): Estudo piloto em adultos jovens brasileiros. Rev. Bras. De Ciência E Mov. 2008, 9, 45–52. [Google Scholar]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.S.; Braggion, G. Questionário Internacional De Atividade Física (Ipaq): Estupo De Validade E Reprodutibilidade No Brasil. Revista Brasileira de Atividade Física Saúde 2012, 6, 5–18. [Google Scholar]
- Heymsfield, S. Human body Composition; Human kinetics: Champaign, IL, USA, 2005; Volume 918. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ceolim, M.F. Padrões de Atividade e de Fragmentação do Sono em Pessoas Idosas. Doctoral Dissertation, University of São Paulo, São Paulo, Brazil, 1999. [Google Scholar]
- Kawakami, N.; Takatsuka, N.; Shimizu, H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004, 27, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.; Draper, N.; Neil, W. Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. Eur. J. Appl. Physiol. 2016, 116, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados; Bookman Editora: Porto Alegre, Brazil, 2009. [Google Scholar]
- Smith, J.J.; Porth, C.M.; Erickson, M. Hemodynamic Response to the Upright Posture. J. Clin. Pharmacol. 1994, 34, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, T.; Niskanen, L.; Geelen, G.; Länsimies, E.; Hartikainen, J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J. Appl. Physiol. 2004, 96, 2333–2340. [Google Scholar] [CrossRef] [Green Version]
- Carnethon, M.R.; Liao, D.; Evans, G.W.; Cascio, W.E.; Chambless, L.E.; Heiss, G. Correlates of the shift in heart rate variability with an active postural change in a healthy population sample: The Atherosclerosis Risk In Communities study. Am. Heart J. 2002, 143, 808–813. [Google Scholar] [CrossRef]
- Acharya, U.R.; Kannathal, N.; Hua, L.M.; Yi, L.M. Study of heart rate variability signals at sitting and lying postures. J. Bodyw. Mov. Ther. 2005, 9, 134–141. [Google Scholar] [CrossRef]
- Gilder, M.; Ramsbottom, R. Change in heart rate variability following orthostasis relates to volume of exercise in healthy women. Auton. Neurosci. 2008, 143, 73–76. [Google Scholar] [CrossRef]
- Chan, H.-L.; Lin, M.-A.; Chao, P.-K.; Lin, C.-H. Correlates of the shift in heart rate variability with postures and walking by time–frequency analysis. Comput. Methods Programs Biomed. 2007, 86, 124–130. [Google Scholar] [CrossRef]
- Chau, J.Y.; Grunseit, A.C.; Chey, T.; Stamatakis, E.; Brown, W.J.; Matthews, C.E.; Bauman, A.E.; Van Der Ploeg, H.P. Daily Sitting Time and All-Cause Mortality: A Meta-Analysis. PLoS ONE 2013, 8, e80000. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Lee, I.-M. Sedentary behaviour and life expectancy in the USA: A cause-deleted life table analysis. BMJ Open 2012, 2, e000828. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Ekelund, U.; Ding, D.; Hamer, M.; Bauman, A.E.; Lee, I.M. Is the time right for quantitative public health guidelines on sitting? A narrative review of sedentary behaviour research paradigms and findings. Br. J. Sports Med. 2019, 53, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Breaks in Sedentary Time: Beneficial associations with metabolic risk. Diabetes Care 2008, 31, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Rees-Punia, E.; Evans, E.M.; Schmidt, M.D.; Gay, J.L.; Matthews, C.E.; Gapstur, S.M.; Patel, A.V. Mortality Risk Reductions for Replacing Sedentary Time With Physical Activities. Am. J. Prev. Med. 2019, 56, 736–741. [Google Scholar] [CrossRef]
- Pereira, S.M.P.; Ki, M.; Power, C. Sedentary Behaviour and Biomarkers for Cardiovascular Disease and Diabetes in Mid-Life: The Role of Television-Viewing and Sitting at Work. PLoS ONE 2012, 7, e31132. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.D.; Zhou, X.; Darwent, D.; Kosmadopoulos, A.; Dawson, D.; Sargent, C.; Roach, G. Are two halves better than one whole? A comparison of the amount and quality of sleep obtained by healthy adult males living on split and consolidated sleep–wake schedules. Anal. Prev. 2017, 99, 428–433. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The Cumulative Cost of Additional Wakefulness: Dose-Response Effects on Neurobehavioral Functions and Sleep Physiology From Chronic Sleep Restriction and Total Sleep Deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Bassett, S.M.; Lupis, S.B.; Gianferante, D.; Rohleder, N.; Wolf, J.M. Sleep quality but not sleep quantity effects on cortisol responses to acute psychosocial stress. Stress 2015, 18, 638–644. [Google Scholar] [CrossRef] [Green Version]
- Simpson, N.S.; Gibbs, E.L.; Matheson, G.O. Optimizing sleep to maximize performance: Implications and recommendations for elite athletes. Scand. J. Med. Sci. Sports 2017, 27, 266–274. [Google Scholar] [CrossRef]
- O’Connell, S.E.; Griffiths, P.L.; Clemes, S.A. Seasonal variation in physical activity, sedentary behaviour and sleep in a sample of UK adults. Ann. Hum. Biol. 2014, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ezeugwu, V.E.; Manns, P.J. Sleep Duration, Sedentary Behavior, Physical Activity, and Quality of Life after Inpatient Stroke Rehabilitation. J. Stroke Cerebrovasc. Dis. 2017, 26, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Kolbe-Alexander, T.; Duncan, M.; Brown, W. Sitting time, physical activity and sleep by work type and pattern—The Australian Longitudinal Study on Women’s Health. Int. J. Environ. Res. Public Health 2017, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Agelink, M.W.; Malessa, R.; Baumann, B.; Majewski, T.; Akila, F.; Zeit, T.; Ziegler, D. Standardized tests of heart rate variability: Normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin. Auton. 2001, 11, 99–108. [Google Scholar] [CrossRef]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-Four Hour Time Domain Heart Rate Variability and Heart Rate: Relations to Age and Gender Over Nine Decades. J. Am. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Volanti, V.M.; Pires, C.S.; Fernandes, F.C. Variabilidade da freqüência cardíaca em diferentes faixas etárias. Revista Brasileira de Fisioterapia 2006, 10, 413. [Google Scholar] [CrossRef]
- Arora, T.; Chen, M.Z.; Omar, O.M.; Cooper, A.R.; Andrews, R.C.; Taheri, S. An investigation of the associations among sleep duration and quality, body mass index and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. Ther. Adv. Endocrinol. Metab. 2016, 7, 3–11. [Google Scholar] [CrossRef]
- Rahe, C.; Czira, M.E.; Teismann, H.; Berger, K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015, 16, 1225–1228. [Google Scholar] [CrossRef]
- Kahlhöfer, J.; Karschin, J.; Breusing, N.; Bosy-Westphal, A. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity 2016, 24, 335–341. [Google Scholar] [CrossRef]

| Variable | Median (IQ 25%–75%) |
|---|---|
| PSQI | 5 (4–6) |
| Physical Activity Level (min/wk) | 440 (300–640) |
| Caloric expenditure in vigorous and moderate intensity physical activity (kcal/wk) | 2356 (1623–3822) † |
| Caloric expenditure in light intensity physical activity (kcal/wk) | 120 (0–450) † |
| Sedentary behavior (min/wk) | 2580 (1800–4260) |
| Variable | Supine | Orthostatic | p |
|---|---|---|---|
| HR (bpm) | 58.11 (54.32–63.92) | 71.93 (67.53–75.20) | <0.001 |
| SBP (mmHg) | 122.0 (115.0–134.0) | 117.0 (107.0–133.0) | 0.013 |
| DBP (mmHg) | 70.0 (64.0–76.0) | 69.0 (64.0–77.0) | 0.825 |
| MAP (mmHg) | 89.33 (80.33–94.0) | 85.33 (78.0–94.0) | 0.261 |
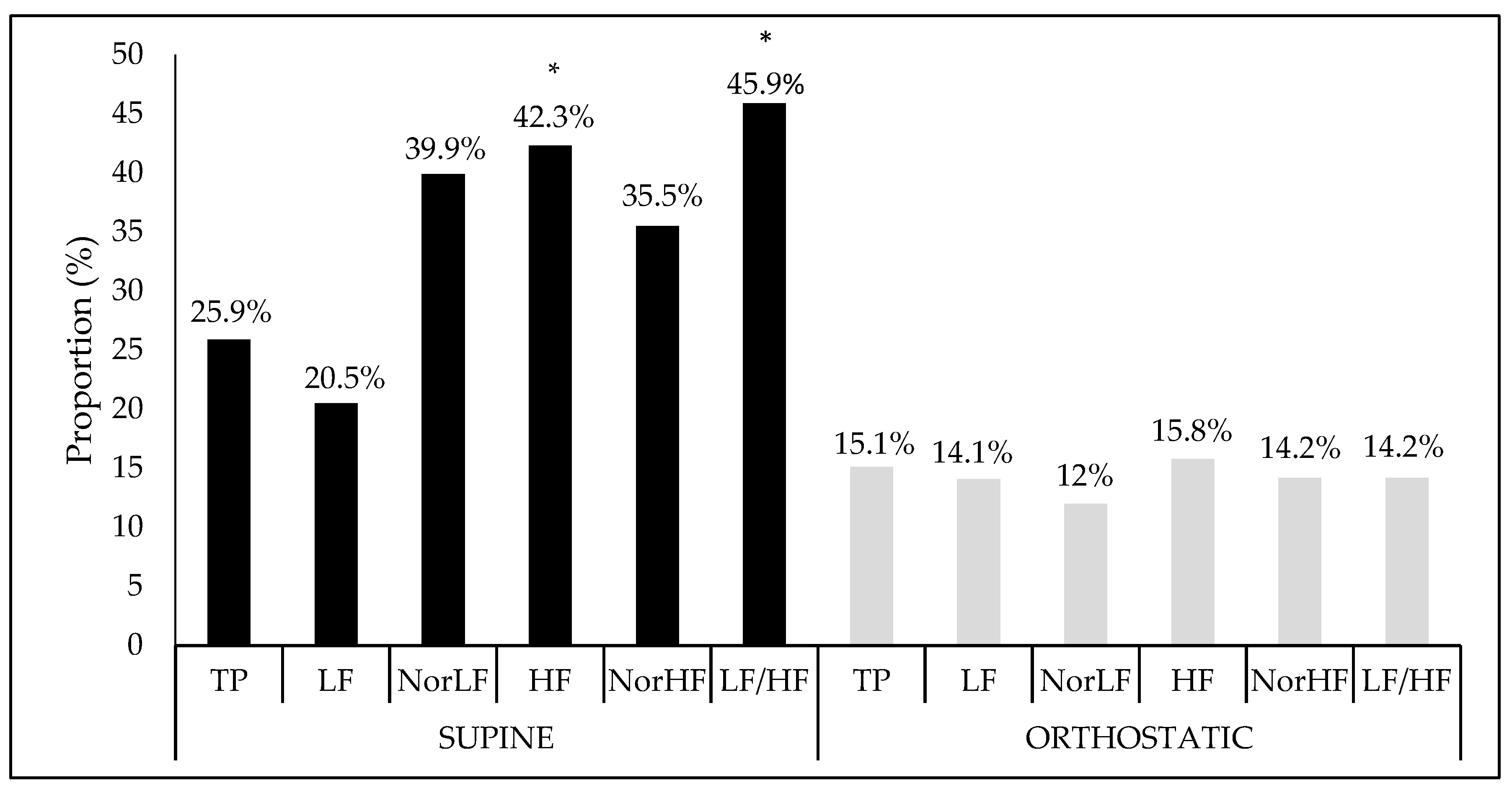
| TP (ms2) | 4120 (2535–7412) | 3943 (2493–6093) | 0.883 |
| LF (ms2) | 1339 (792–2288) | 2170 (1122–3608) * | 0.005 |
| NorLF (%) | 52 (40.8–67.5) | 86 (77.8–91.9) * | <0.001 |
| HF (ms2) | 1237 (448–2184) | 328 (193–715) * | <0.001 |
| NorHF (%) | 47.9 (32.2–59.1) | 14 (8.1–22.1) * | <0.001 |
| LF/HF | 1.12 (0.76–2.37) | 6.16 (3.52–11.38) * | <0.001 |
| Variable | EKcal Vig-Mod (Kcal/Wk) | EKcal Light (Kcal/Wk) | Sedentary Behavior (Min/Wk) | Sleep Quality (PSQI) | ||||
|---|---|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | Beta | p | |
| SUPINE | ||||||||
| TP (ms2) | 0.08 | 0.54 | −0.16 | 0.25 | −0.44 | 0.00 * | 0.19 | 0.20 |
| LF (ms2) | 0.01 | 0.97 | −0.19 | 0.20 | −0.28 | 0.07 | 0.07 | 0.64 |
| NorLF (%) | 0.09 | 0.55 | −0.13 | 0.37 | 0.31 | 0.04 * | −0.12 | 0.42 |
| HF (ms2) | −0.07 | 0.59 | −0.09 | 0.46 | −0.63 | 0.00 * | 0.22 | 0.09 |
| NorHF (%) | −0.05 | 0.71 | −0.02 | 0.91 | −0.38 | 0.01 * | 0.09 | 0.54 |
| LF/HF | 0.10 | 0.45 | −0.10 | 0.44 | 0.53 | 0.00 * | −0.21 | 0.12 |
| ORTHOSTATIC | ||||||||
| TP (ms2) | 0.17 | 0.24 | −0.12 | 0.40 | −0.21 | 0.17 | 0.10 | 0.50 |
| LF (ms2) | 0.22 | 0.13 | −0.05 | 0.74 | −0.26 | 0.09 | 0.13 | 0.39 |
| NorLF (%) | 0.09 | 0.55 | −0.13 | 0.37 | 0.31 | 0.04 * | −0.12 | 0.42 |
| HF (ms2) | −0.02 | 0.89 | −0.18 | 0.23 | −0.28 | 0.07 | 0.02 | 0.89 |
| NorHF (%) | −0.23 | 0.13 | −0.14 | 0.34 | −0.10 | 0.52 | −0.07 | 0.66 |
| LF/HF | 0.23 | 0.11 | 0.14 | 0.33 | 0.15 | 0.33 | 0.05 | 0.73 |
| Variables | EKcal Vig-Mod (Kcal/Wk) | EKcal Light (Kcal/Wk) | Sedentary Behavior (Min/Wk) | Sleep Quality (PSQI) | ||||
|---|---|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | Beta | p | |
| SUPINE | ||||||||
| TP (ms2) | 0.15 | 0.30 | −0.17 | 0.22 | −0.58 | 0.03 * | 0.14 | 0.33 |
| LF (ms2) | 0.09 | 0.55 | −0.17 | 0.25 | −0.50 | 0.06 | 0.01 | 0.96 |
| NorLF (%) | 0.12 | 0.33 | −0.01 | 0.97 | −0.24 | 0.29 | −0.22 | 0.11 |
| HF (ms2) | −0.03 | 0.78 | −0.14 | 0.27 | −0.50 | 0.03 * | 0.22 | 0.09 |
| NorHF (%) | −0.10 | 0.45 | −0.12 | 0.35 | 0.12 | 0.62 | 0.18 | 0.19 |
| LF/HF | 0.15 | 0.22 | −0.01 | 0.92 | 0.09 | 0.69 | −0.30 | 0.02 * |
| ORTHOSTATIC | ||||||||
| TP (ms2) | 0.23 | 0.13 | −0.08 | 0.60 | −0.56 | 0.43 | 0.03 | 0.84 |
| LF (ms2) | 0.24 | 0.11 | −0.01 | 0.97 | −0.52 | 0.06 | 0.08 | 0.61 |
| NorLF (%) | 0.19 | 0.22 | 0.17 | 0.26 | 0.07 | 0.79 | 0.22 | 0.17 |
| HF (ms2) | 0.02 | 0.88 | −0.15 | 0.33 | −0.41 | 0.13 | −0.02 | 0.92 |
| NorHF (%) | −0.19 | 0.19 | −0.14 | 0.34 | −0.01 | 0.97 | −0.07 | 0.66 |
| LF/HF | 0.21 | 0.16 | 0.13 | 0.38 | 0.11 | 0.69 | 0.06 | 0.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, R.R.; Rosa, E.C.; Rosa, T.; Ferreira, E.A.; Gris, E.F.; de Andrade, R.V.; Amato, A.A. Sedentary Behavior: A Key Component in the Interaction between an Integrated Lifestyle Approach and Cardiac Autonomic Function in Active Young Men. Int. J. Environ. Res. Public Health 2019, 16, 2156. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16122156
dos Santos RR, Rosa EC, Rosa T, Ferreira EA, Gris EF, de Andrade RV, Amato AA. Sedentary Behavior: A Key Component in the Interaction between an Integrated Lifestyle Approach and Cardiac Autonomic Function in Active Young Men. International Journal of Environmental Research and Public Health. 2019; 16(12):2156. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16122156
Chicago/Turabian Styledos Santos, Renan R., Erica C. Rosa, Thiago Rosa, Eduardo A. Ferreira, Eliana F. Gris, Rosângela V. de Andrade, and Angélica A. Amato. 2019. "Sedentary Behavior: A Key Component in the Interaction between an Integrated Lifestyle Approach and Cardiac Autonomic Function in Active Young Men" International Journal of Environmental Research and Public Health 16, no. 12: 2156. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16122156




