Treatment with 3-Aminobenzamide Negates the Radiofrequency-Induced Adaptive Response in Two Cell Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. RF Exposure and Dosimetry
2.2. Experimental Protocol and Culture Setup
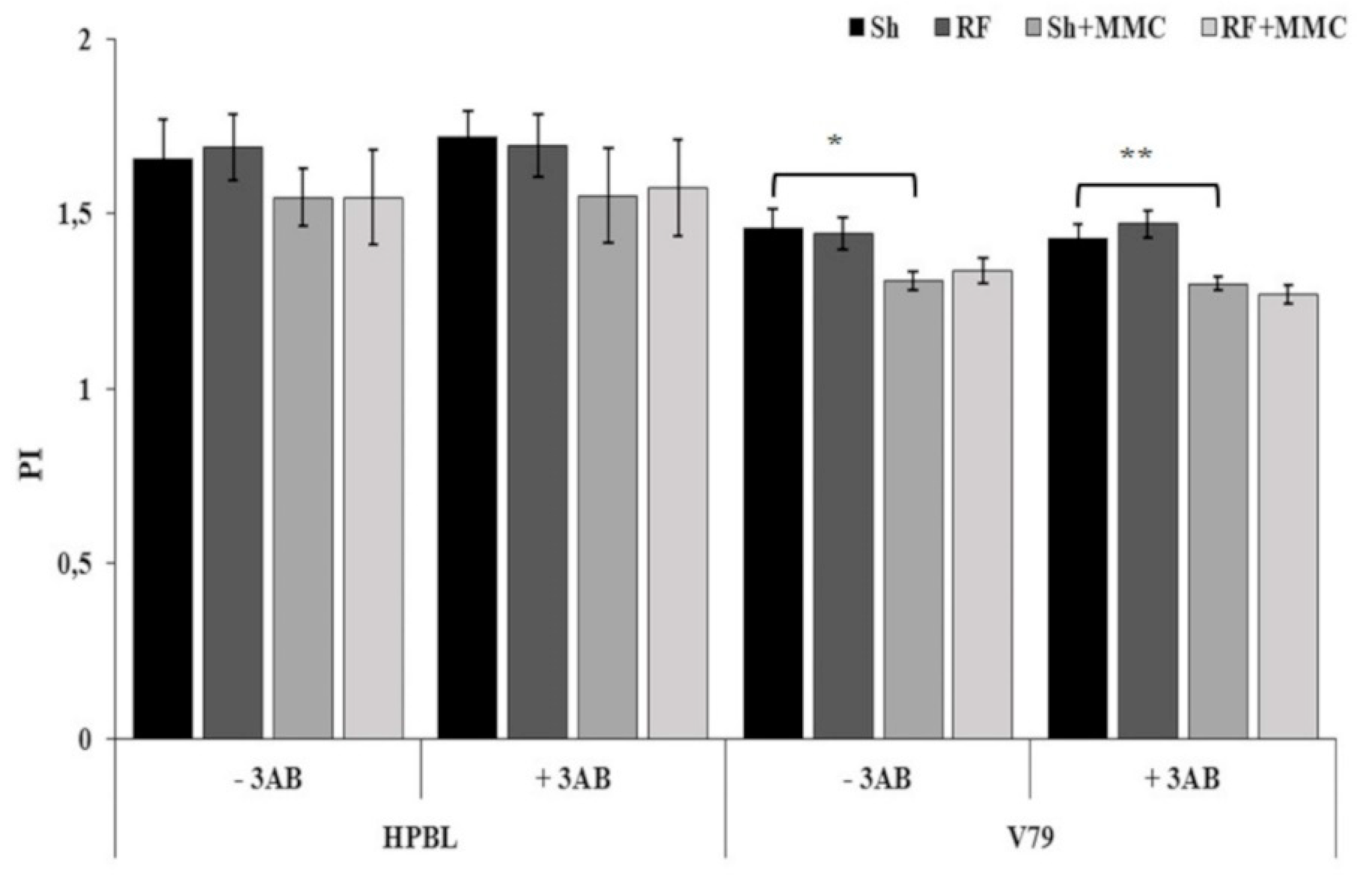
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joiner, M.C.; Lambin, P.; Marples, B. Adaptive response and induced resistance. C. R. Acad. Sci. III 1999, 322, 167–175. [Google Scholar] [CrossRef]
- Stecca, C.; Gerber, G.B. Adaptive response to DNA-damaging agents: A review of potential mechanisms. Biochem. Pharmacol. 1998, 55, 941–951. [Google Scholar] [CrossRef]
- Dimova, E.G.; Bryant, P.E.; Chankova, S.G. “Adaptive response”—Some underlying mechanisms and open questions. Genet. Mol. Biol. 2008, 31, 396–408. [Google Scholar] [CrossRef]
- Sannino, A.; Sarti, M.; Reddy, S.B.; Prihoda, T.J.; Vijayalaxmi; Scarfi, M.R. Induction of adaptive response in human blood lymphocytes exposed to radiofrequency radiation. Radiat. Res. 2009, 171, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Zeni, O.; Sarti, M.; Romeo, S.; Reddy, S.B.; Belisario, M.A.; Prihoda, T.J.; Vijayalaxmi; Scarfi, M.R. Induction of adaptive response in human blood lymphocytes exposed to 900 MHz radiofrequency fields: Influence of cell cycle. Int. J. Radiat. Biol. 2011, 87, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Zeni, O.; Sannino, A.; Romeo, S.; Massa, R.; Sarti, M.; Reddy, A.B.; Prihoda, T.J.; Vijayalaxmi; Scarfi, M.R. Induction of an adaptive response in human blood lymphocytes exposed to radiofrequency fields: Influence of the universal mobile telecommunication system (UMTS) signal and the specific absorption rate. Mutat. Res. 2012, 747, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Zeni, O.; Romeo, S.; Massa, R.; Gialanella, G.; Grossi, G.; Manti, L.; Vijayalaxmi; Scarfi, M.R. Adaptive response in human blood lymphocytes exposed to non-ionizing radiofrequency fields: Resistance to ionizing radiation-induced damage. J. Radiat. Res. 2014, 55, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Zeni, O.; Romeo, S.; Massa, R.; Scarfi, M.R. Adverse and beneficial effects in Chinese hamster lung fibroblast cells following radiofrequency exposure. Bioelectromagnetics 2017, 38, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Sannino, A.; Romeo, S.; Zeni, O.; Santini, S.J.; Rispoli, R.; Amicarelli, F.; Scarfì, M.R. Protective effect of 1950 MHz electromagnetic field in human neuroblastoma cells challenged with menadione. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Romeo, S.; Sannino, A.; Zeni, O.; Angrisani, L.; Massa, R.; Scarfi, M.R. Effects of Radiofrequency Exposure and Co-Expo-sure on Human Lymphocytes: The Influence of Signal Modulation and Bandwidth. IEEE J.-ERM 2019, in press. [Google Scholar] [CrossRef]
- Vijayalaxmi; Cao, Y.; Scarfi, M.R. Adaptive response in mammalian cells exposed to non-ionizing radiofrequency fields: A review and gaps in knowledge. Mutat. Res. 2014, 760, 36–45. [Google Scholar] [CrossRef]
- Zong, C.; Ji, Y.; He, Q.; Zhu, S.; Qin, F.; Tong, J.; Cao, Y. Adaptive response in mice exposed to 900 MHZ radiofrequency fields: Bleomycin-induced DNA and oxidative damage/repair. Int. J. Radiat. Biol. 2015, 91, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; He, Q.; Sun, Y.; Tong, J.; Cao, Y. Adaptive response in mouse bone-marrow stromal cells exposed to 900-MHz radiofrequency fields: Gamma-radiation-induced DNA strand breaks and repair. J. Toxicol. Environ. Health. PT A 2016, 79, 419–426. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Sun, Y.; Zong, L.; Tong, J.; Cao, Y. Induction of Poly(ADP-ribose) Polymerase in Mouse Bone Marrow Stromal Cells Exposed to 900 MHz Radiofrequency Fields: Preliminary Observations. Biomed. Res. Int. 2016, 2016, 4918691. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zong, L.; Sun, Y.; Vijayalaxmi; Prihoda, T.J.; Tong, J.; Cao, Y. Adaptive response in mouse bone marrow stromal cells exposed to 900MHz radiofrequency fields: Impact of poly (ADP-ribose) polymerase (PARP). Mutat. Res. 2017, 820, 19–25. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, D.; Desnoyers, S.; D’Silva, I.; Poirier, G.G. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 1999, 342 Pt 2, 249–268. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.M.; Cortes, U.; Wang, Z.Q. Poly(ADP-ribose) polymerase: A guardian angel protecting the genome and suppressing tumorigenesis. Biochim. Biophys. Acta. 2001, 1552, 27–37. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.C.; de Murcia, G. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell. Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Hassa, P.O.; Hottiger, M.O. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 2008, 13, 3046–3082. [Google Scholar] [CrossRef]
- Fenech, M.; Morley, A.A. Cytokinesis-block micronucleus method in human lymphocytes: Effect of in vivo ageing and low dose X-irradiation. Mutat. Res. 1986, 161, 193–198. [Google Scholar] [CrossRef]
- Romeo, S.; D’Avino, C.; Pinchera, D.; Zeni, O.; Scarfi, M.R.; Massa, R. A Waveguide Applicator for In Vitro Exposures to Single or Multiple ICT Frequencies. IEEE Trans. Microw. Theory Tech. 2013, 61, 1994–2004. [Google Scholar] [CrossRef]
- Sannino, A.; Calabrese, M.L.; d’Ambrosio, G.; Massa, R.; Petraglia, G.; Mita, P.; Sarti, M.; Scarfi, M.R. Evaluation of cytotoxic and genotoxic effects in human peripheral blood leukocytes following exposure to 1950-MHz modulated signal. IEEE Trans. Plasma Sci. 2006, 34, 1441–1448. [Google Scholar] [CrossRef]
- Zeni, O.; Sannino, A.; Romeo, S.; Micciulla, F.; Bellucci, S.; Scarfi, M.R. Growth inhibition, cell-cycle alteration and apoptosis in stimulated human peripheral blood lymphocytes by multiwalled carbon nanotube buckypaper. Nanomedicine 2015, 10, 351–360. [Google Scholar] [CrossRef]
- Vijayalaxmi; Burkart, W. Effect of 3-aminobenzamide on chromosome damage in human blood lymphocytes adapted to bleomycin. Mutagenesis 1989, 4, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M.; Bonassi, S.; Turner, J.; Lando, C.; Ceppi, M.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Bigatti, M.P.; et al. Intra- and inter-laboratory variation in the scoring of micronuclei and nucleoplasmic bridges in binucleated human lymphocytes. Results of an international slide-scoring exercise by the HUMN project. Mutat. Res. 2003, 534, 45–64. [Google Scholar] [CrossRef]
- Bose Girigoswami, K.; Bhaumik, G.; Ghosh, R. Induced resistance in cells exposed to repeated low doses of H2O2 involves enhanced activity of antioxidant enzymes. Cell. Biol. Int. 2005, 29, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Bodycote, J.; Wolff, S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Leal, B.Z.; Deahl, T.S.; Meltz, M.L. Variability in adaptive response to low dose radiation in human blood lymphocytes: Consistent results from chromosome aberrations and micronuclei. Mutat. Res. 1995, 348, 45–50. [Google Scholar] [CrossRef]
- Krishnaja, A.P.; Sharma, N.K. Variability in cytogenetic adaptive response of cultured human lymphocytes to mitomycin C, bleomycin, quinacrine dihydrochloride, Co60 gamma-rays and hyperthermia. Mutagenesis 2008, 23, 77–86. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sannino, A.; Zeni, O.; Romeo, S.; Lioi, M.B.; Scarfì, M.R. Treatment with 3-Aminobenzamide Negates the Radiofrequency-Induced Adaptive Response in Two Cell Models. Int. J. Environ. Res. Public Health 2019, 16, 2768. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152768
Sannino A, Zeni O, Romeo S, Lioi MB, Scarfì MR. Treatment with 3-Aminobenzamide Negates the Radiofrequency-Induced Adaptive Response in Two Cell Models. International Journal of Environmental Research and Public Health. 2019; 16(15):2768. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152768
Chicago/Turabian StyleSannino, Anna, Olga Zeni, Stefania Romeo, Maria Brigida Lioi, and Maria Rosaria Scarfì. 2019. "Treatment with 3-Aminobenzamide Negates the Radiofrequency-Induced Adaptive Response in Two Cell Models" International Journal of Environmental Research and Public Health 16, no. 15: 2768. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152768





