Aggravation of Human Diseases and Climate Change Nexus
Abstract
:1. Introduction
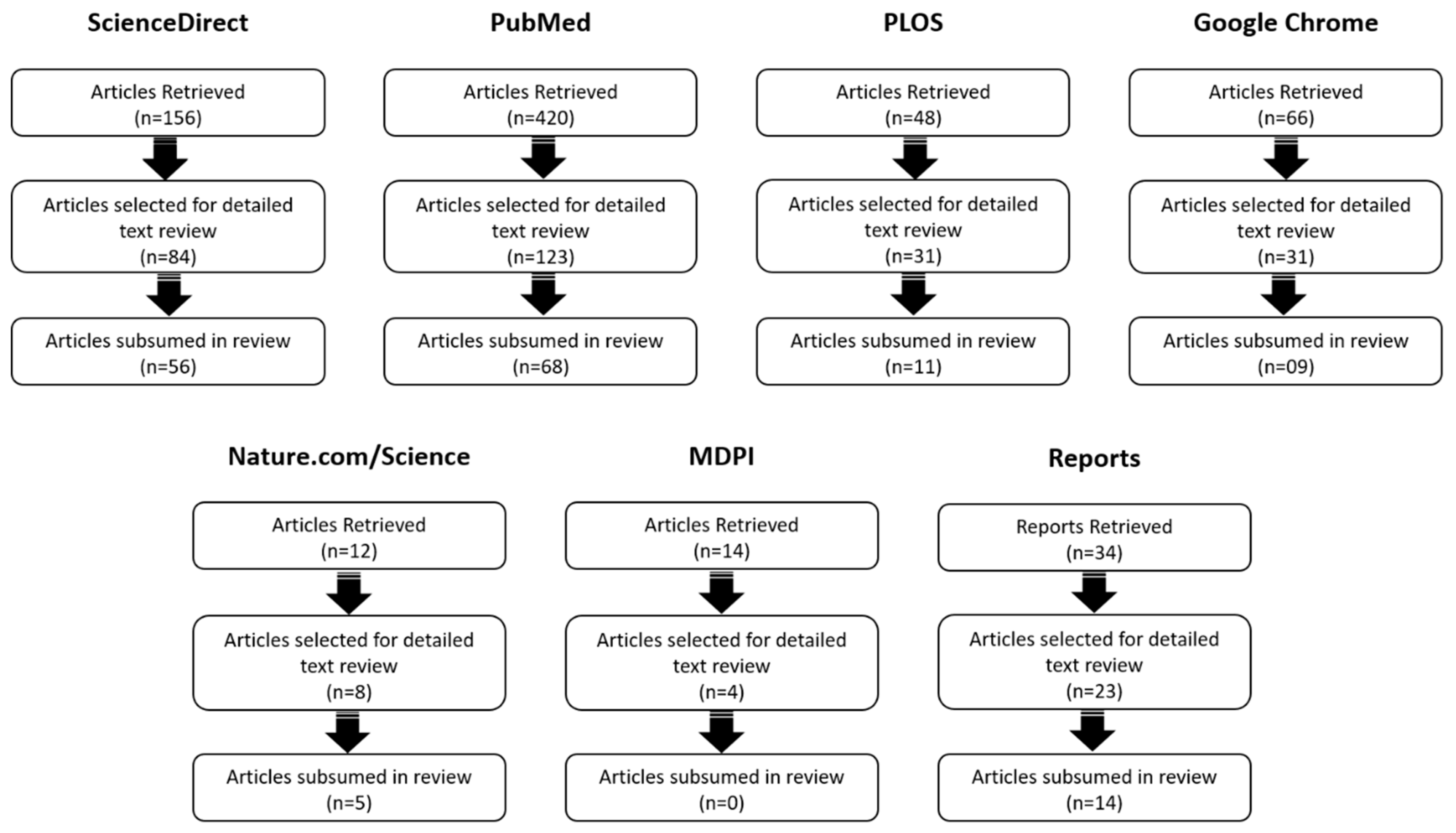
2. Methods
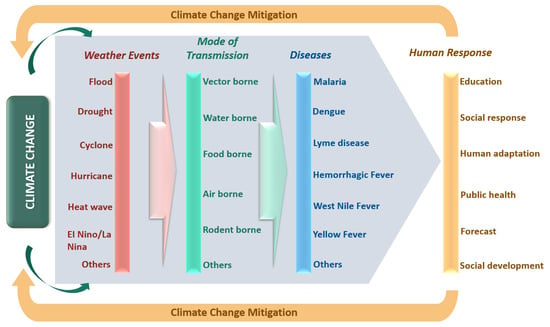
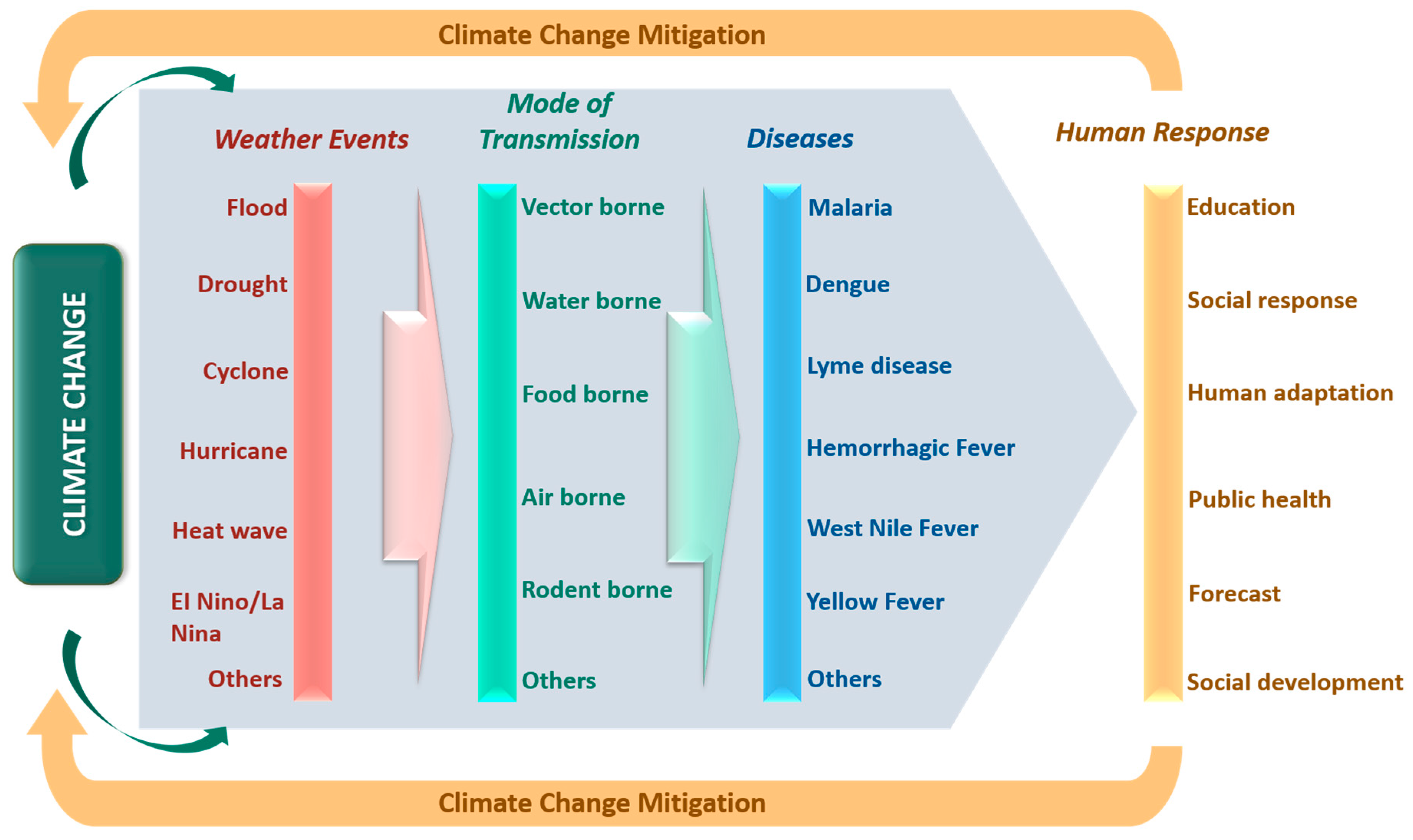
3. Extreme Weather Events
4. Relationship Between Climate Change and Transmissible Diseases
4.1. Impact on Pathogens
4.2. Impacts on Vectors/Hosts
4.2.1. Effect of Spatiotemporal Distribution of Vectors
4.2.2. Effect on Mortality Rate
4.3. Mode of Transmission
5. Need for Urgent Human Response
5.1. Climate Change and Public Health System
5.2. Climate Change and Adaptation Measures
5.3. Climate Change and Adaptation Policy
5.4. Climate Change and Social Development
6. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMichael, A.J. Globalization, climate change, and human health. N. Engl. J. Med. 2013, 368, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M. Summary for policymakers. Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change (IPCC). In Climate Change 2007: The Physical Science Basis; Miller, H.L., Ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Available online: https://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg1_report_the_physical_science_basis.htm (accessed on 2 August 2018).
- Christiansen, T.; Voigt, T. Impact of Europe’s Changing Climate—2008 Indicator-Based Assessment. In Joint EEA-JRC-WHO Report; European Environment Agency: Copenhagen, Denmark, 2008; Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/jrc_reference_report_2008_09_climate_change.pdf (accessed on 2 August 2018).
- Watson, R.T. A Contribution of Working Groups I, II, and III to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change (IPCC). In Climate Change 2001: Synthesis Report; Team, C.W., Ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; Available online: https://www.ipcc.ch/pdf/climate-changes-2001/synthesis-syr/english/front.pdf (accessed on 2 August 2018).
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, P.R. Is global warming harmful to health? Sci. Am. 2000, 283, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001, 3, 747–754. [Google Scholar] [CrossRef]
- Rigby, C.W.; Rosen, A.; Berry, H.L.; Hart, C.R. If the land’s sick, we’re sick: The impact of prolonged drought on the social and emotional well-being of Aboriginal communities in rural New South Wales. Aust. J. Rural Health 2011, 19, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Sartore, G.; Hoolahan, B.; Tonna, A.; Kelly, B.; Stain, H. Wisdom from the drought: Recommendations from a consultative conference. Aust. J. Rural Health 2005, 13, 315–320. [Google Scholar] [CrossRef]
- Wu, X.X.; Tian, H.Y.; Zhou, S.; Chen, L.F.; Xu, B. Impact of global change on transmission of human infectious diseases. Sci. China Earth Sci. 2014, 57, 189–203. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Zhuang, J.L.; Yang, M.X.; Zhang, Z.J.; Wei, J.G.; Peng, W.X.; Zhao, G.M.; Zhang, S.M.; Jiang, Q.W. Effects of low temperature on the schistosome-transmitting snail Oncomelania hupensis and the implications of global climate change. Molluscan Res. 2010, 30, 102–108. [Google Scholar]
- Henderson, A.; Leake, C.J.; Burke, D.S. Japanese encephalitis in Nepal. Lancet 1983, 2, 1359–1360. [Google Scholar] [CrossRef]
- Bista, M.B.; Shrestha, J.M. Epidemiological situation of Japanese encephalitis in Nepal. J. Nepal. Med. Assoc. 2005, 44, 51–56. [Google Scholar] [CrossRef]
- Impoinvil, D.E.; Solomon, T.; Schluter, W.W.; Rayamajhi, A.; Bichha, R.P.; Shakya, G.; Caminade, C.; Baylis, M. The spatial heterogeneity between Japanese encephalitis incidence distribution and environmental variables in Nepal. PLoS ONE 2011, 6, e22192. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.D.; Sharma, M.; Bhandari, S. Visceral leishmaniasis in Nepal during 1980–2006. J. Commun. Dis. 2006, 38, 139–148. [Google Scholar] [PubMed]
- Pun, S.B.; Sato, T.; Pandey, K.; Pandey, B.D. Changing trends in visceral leishmaniasis: 10 years’ experience at a referral hospital in Nepal. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 550–554. [Google Scholar] [CrossRef]
- Department of Health Services. Annual Report 2069/70 (2012/2013) Kathmandu: Department of Health Services, Ministry of Health and Population, Government of Nepal; Department of Health Services: Kathmandu, Nepal, 2014. Available online: http://dohs.gov.np/wp-content/uploads/2014/04/Annual_Report_2069_70.pdf (accessed on 2 August 2018).
- Adhikari, R.K.; Bhusal, K.P. Surveillance of lymphatic filariasis in selected districts of Nepal. J. Inst. Med. 2008, 30, 35–40. [Google Scholar]
- Slaper, H.; Velders, G.J.M.; Matthijsen, J. Ozone depletion and skin cancer incidence: A source risk approach. J. Hazard Mater. 1998, 61, 77–84. [Google Scholar] [CrossRef]
- Verbrugge, L.M.; Rainey, J.J.; Reimink, R.L.; Blankespoor, H.D. Swimmer’s itch: Incidence and risk factors. Am. J. Public Health. 2004, 94, 738–741. [Google Scholar] [CrossRef]
- Cheng, A.; Currie, B. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [CrossRef]
- Kuhn, K.; Campbell-Lendrum, D.; Haines, A.; Cox, J. Using Climate to Predict Infectious Diseases Epidemics; World Health Organization: Geneva, Switzerland, 2005; Available online: http://www.who.int/globalchange/publications/infectdiseases/en/ (accessed on 2 August 2018).
- Antia, R.; Regoes, R.; Koella, J.; Bergstrom, C. The role of evolution in the emergence of infectious diseases. Nature 2013, 426, 658–661. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, V.; Stocker, T.F.; Qin, D.; Dokken, D.J.; Ebi, K.L.; Mastrandrea, M.D.; Mach, K.J.; Plattner, G.-K.; Allen, S.K.; Tignor, M.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Midgley, P.M., Ed.; Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; Available online: https://www.ipcc.ch/pdf/special-reports/srex/SREX_Full_Report.pdf (accessed on 8 August 2018).
- Zhu, Y.; Toth, Z. Extreme weather events and their probabilistic prediction by the NCEP ensemble forecast system. In Proceedings of the 81st American Meteorological Society Annual Meeting, Albuquerque, NM, USA, 14–19 January 2001; Available online: https://ams.confex.com/ams/annual2001/webprogram/Paper17656.html (accessed on 4 August 2018).
- Lubchenco, J.; Karl, T.R. Predicting and managing extreme weather events. Phys. Today 2012, 65, 31–37. [Google Scholar] [CrossRef]
- Nicholls, N.E. El Nino-Southern Oscillation and vector-borne disease. Lancet 1993, 342, 1284–1285. [Google Scholar] [CrossRef]
- Hay, S.I.; Cox, J.; Rogers, D.J.; Randolph, S.E.; Stern, D.I.; Shanks, G.D.; Myers, M.F.; Snow, R.W. Climate change and the resurgence of malaria in the East African highlands. Nature 2002, 415, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Shanks, G.D.; Hay, S.I.; Stern, D.I.; Biomndo, K.; Snow, R.W. Meteorologic influences on Plasmodium falciparum malaria in the highland tea estates of Kericho, western Kenya. Emerg. Infect. Dis. 2002, 8, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Linthicum, K.J.; Anyamba, A.; Tucker, C.J.; Kelley, P.W.; Myers, M.F.; Peters, C.J. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 1999, 285, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G.; Linthicum, K.J.; James, A.D. Rainfall and epizootic Rift Valley fever. Bull. World Health Organ. 1985, 63, 941–943. [Google Scholar] [PubMed]
- McMichael, A.J.; Lindgren, E. Climate change: Present and future risks to health, and necessary responses. J. Intern. Med. 2011, 270, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.E.; Hess, J.J. Climate change. Impacts on and implications for global health. Am. J. Prev. Med. 2008, 35, 527–538. [Google Scholar] [CrossRef]
- Haines, A.; Kovats, R.S.; Campbell-Lendrum, D.; Corvalan, C. Climate change and human health: Impacts, vulnerability, and mitigation. Lancet 2006, 367, 2101–2109. [Google Scholar] [CrossRef]
- Haines, A.; Kovats, R.S.; Campbell-Lendrum, D.; Corvalan, C. Climate change and human health: Impacts, vulnerability and public health. Public Health 2006, 120, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Dobson, A.J.; McElduff, P.; Salomaa, V.; Kuulasmaa, K.; Sans, S.; WHO MONICA Project. Cold periods and coronary events: An analysis of populations worldwide. Epidemiol. Community Health 2005, 59, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Meehl, G.A.; Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramon, M.; Zanobetti, A.; Cavanagh, D.P.; Schwartz, J. Extreme temperatures and mortality: Assessing effect modification by personal characteristics and specific cause of death in a multicity case-only analysis. Environ. Health Perspect. 2006, 114, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, M.G.; Corrao, S.; Biondo, G.B.; Renda, N.; Braschi, A.; Novo, G.; Di Girolamo, A.; Braschi, G.B.; Novo, S. Influence of climatic variables on acute myocardial infarction hospital admissions. Int. J. Cardiol. 2009, 137, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Mireku, N.; Wang, Y.; Ager, J.; Reddy, R.C.; Baptist, A.P. Changes in weather and the effects on pediatric asthma exacerbations. Ann. Allergy Asthma Immunol. 2009, 103, 220–224. [Google Scholar] [CrossRef]
- Lin, S.; Luo, M.; Walker, R.J.; Liu, X.; Hwang, S.A.; Chinery, R. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology 2009, 20, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.B.; Tebaldi, C. Increasing prevalence of extreme summer temperatures in the US. Clim. Chang. 2012, 111, 487–495. [Google Scholar] [CrossRef]
- Ganguly, A.R.; Steinhaeuser, K.; Erickson, D.J.; Branstetter, M.; Parish, E.S.; Singh, N.; Drake, J.B.; Buja, L. Higher trends but larger uncertainty and geographic variability in 21st century temperature and heat waves. Proc. Natl. Acad. Sci. USA 2009, 106, 15555–15559. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Heat-related deaths—Four states, July–August 2001, and United States, 1979–1999. Morb. Mortal. Wkly. Rep. 2002, 51, 567–570. [Google Scholar]
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of ambient temperature on the incidence of myocardial infarction. Heart 2009, 95, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-H.; Hong, Y.-C.; Kim, H. Effects of diurnal temperature range on cardiovascular and respiratory hospital admissions in Korea. Sci. Total Environ. 2012, 417–418, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X. The influence of flood and drought on the epidemic of hemorrhagic fever with renal syndrome and prevention measures. Chin. J. Public Health 1999, 15, 665. [Google Scholar]
- Pan, H.M.; Cheng, D.M.; Shi, Y.N. The influence of flood disasters to the leptospirosis epidemic. Chin. J. Nat. Med. 2003, 5, 73–75. [Google Scholar]
- Sanders, E.J.; Rigau-Perez, J.G.; Smits, H.L.; Deseda, C.C.; Vorndam, V.A.; Aye, T.; Spiegel, R.A.; Weyant, R.S.; Bragg, S.L. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996. Am. J. Trop. Med. Hyg. 1999, 61, 399–404. [Google Scholar] [CrossRef]
- Shultz, J.M.; Russel, J.; Espinel, Z. Epidemiology of tropical cyclones: The dynamics of disaster, disease, and development. Epidemiol. Rev. 2005, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Brummel, S.; Wu, J.; Stern, H.; Ostro, B.; Lipsett, M.; Winer, A.; Street, D.H.; Zhang, L.; Tjoa, T.; et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup. Environ. Med. 2009, 66, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, D.A.; Wigder, N.L. Ozone production from wildfires: A critical review. Atmos. Environ. 2012, 51, 1–10. [Google Scholar] [CrossRef]
- Ebi, K.L.; Exuzides, K.A.; Lau, E.; Kelsh, M.; Barnston, A. Association of normal weather periods and El Nino events with hospitalization for viral pneumonia in females: California, 1983–1998. Am. J. Public Health 2001, 91, 1200–1208. [Google Scholar] [CrossRef]
- Chretien, J.P.; Anyamba, A.; Bedno, S.A.; Breiman, R.F.; Sang, R.; Sergon, K.; Powers, A.M.; Onyango, C.O.; Small, J.; Tucker, C.J. Drought-associated Chikungunya emergence along coastal East Africa. Am. J. Trop. Med. Hyg. 2007, 76, 405–407. [Google Scholar] [CrossRef]
- Dwight, R.H.; Baker, D.B.; Semenza, J.C.; Olson, B.H. Health effects associated with recreational water use: Urban versus rural California. Am. J. Public Health 2004, 94, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2010, 2, 45–65. [Google Scholar] [CrossRef]
- Reacher, M.; McKenzie, K.; Lane, C.; Nichols, T.; Kedge, I.; Iversen, A.; Hepple, P.; Walter, T.; Laxton, C.; Simpson, J. Health impacts of flooding in Lewes: A comparison of reported gastrointestinal and other illness and mental health in flooded and non-flooded households. Commun. Dis. Public Health 2004, 7, 39–46. [Google Scholar] [PubMed]
- Hajat, S.; O’Connor, M.; Kosatsky, T. Health effects of hot weather: from awareness of risk factors to effective health protection. The Lancet 2010, 375, 856–863. [Google Scholar] [CrossRef]
- Van Doorn, H.R. Emerging infectious diseases. Medicine 2017, 45, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human diseases emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Mellor, P.S.; Leake, C.J. Climate and geographic influences on arboviral infection and vectors. Rev. Sci. Tech. 2000, 19, 41–54. [Google Scholar] [CrossRef]
- Tian, H.Y.; Bi, P.; Cazelles, B.; Zhou, S.; Huang, S.Q.; Yang, J.; Pei, Y.; Wu, X.X.; Fu, S.H.; Tong, S.L.; et al. How environmental conditions impact mosquito ecology and Japanese encephalitis: An eco-epidemiological approach. Environ. Int. 2015, 79, 17–24. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Landrigan, C.P.; McMichael, A.J.; Epstein, P.R. The impact of climate change on child health. Ambul. Pediatr. 2003, 3, 44–52. [Google Scholar] [CrossRef]
- Perencevich, E.N.; McGregor, J.C.; Shardell, M.; Furuno, J.P. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect. Control Hosp. Epidemiol. 2008, 29, 1124–1131. [Google Scholar] [CrossRef]
- Anderson, D.J.; Herve, R.; Chen, L.F.; Spelman, D.W.; Hung, Y.; Huang, A.T.; Sexton, D.J.; Raoult, D. Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J. Infect. Dis. 2008, 197, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Epstein, P.R.; Burke, T.A.; Balbus, J.M. Global climate change and emerging infectious diseases. J. Am. Med. Assoc. 1996, 275, 217–223. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Littman, M.; Alpers, K.; Hallauer, J. Vibrio vulnificus wound infections after contact with the Baltic Sea, Germany. Eur. Surg. 2006, 11, E060817. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.J.; Cole, D.J.; Lipp, E.K. Distribution, diversity, and seasonality of waterborne Salmonellae in a rural watershed. Appl. Environ. Microbiol. 2009, 75, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.; McDonald, A.T. Predicting coliform concentrations in upland impoundments: Design and calibration of a multivariate model. Appl. Environ. Microbiol. 1983, 46, 611–618. [Google Scholar] [PubMed]
- El-Fadel, M.; Ghanimesh, S.; Maroun, R.; Alameddine, I. Climate change and temperature rise: Implications on food- and water-borne diseases. Sci. Total Environ. 2012, 437, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wilby, R.L.; Hedger, M.; Orr, H. Climate change impacts and adaptation: A science agenda for the Environmental Agency of England and Wales. Weather 2005, 60, 206–211. [Google Scholar] [CrossRef]
- Jofre, J.; Blanch, A.R.; Lucena, F.; Sabater, S. Water-borne infectious disease outbreaks associated with water scarcity and rainfall events. In Water Scarcity in the Mediterranean: Perspectives Under Global Change; Barcelo, D., Ed.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 8, pp. 147–159. ISBN 978-3-642-03971-3. [Google Scholar]
- Xu, B.; Jin, Z.Y.; Jiang, Z.B.; Guo, J.P.; Timberlake, M.; Ma, X.L.; Weng, Q. Climatological and geographical impacts on global pandemic of influenza A (H1N1). In Global Urban Monitoring and Assessment through Earth Observation, 1st ed.; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781466564503. [Google Scholar]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.M.; Aye, K.M.; Thein, S. The effect of temperature and humidity on dengue virus propagation in Aedes aegypti mosquitos. Southeast Asian J. Trop. Med. Public Health 1998, 29, 280–284. [Google Scholar] [PubMed]
- Chen, P.S.; Tsai, F.T.; Lin, C.K.; Yang, C.Y.; Chan, C.C.; Young, C.Y.; Lee, C.H. Ambient influenza and avian influenza virus during dust storm days and background days. Environ. Health Perspect. 2010, 118, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109, 141–161. [Google Scholar] [CrossRef]
- Mills, J.N.; Gage, K.L.; Khan, A.S. Potential influence of climate change on vector-borne and zoonotic diseases: A review and proposed research plan. Environ. Health Perspect. 2010, 118, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Randolph, S.E. Climate change and vector-borne diseases. Adv. Parasitol. 2006, 62, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Liu-Helmersson, J.; Quam, M.; Wilder-Smith, A.; Stenlund, H.; Ebi, K.; Massad, E.; Rocklov, J. Climate change and Aedes Vectors: 21st century projections for dengue transmission in Europe. EBioMedicine 2016, 7, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, U.B.; Gautam, S.; Bawa, K.S. Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS ONE 2012, 7, e36741. [Google Scholar] [CrossRef]
- Sakya, G.M. Present Status of Malaria in Nepal. J. Nepal Med. Assoc. 1981, 19, 21–28. [Google Scholar] [CrossRef]
- Dhimal, M.; Ahrens, B.; Kuch, U. Malaria control in Nepal 1963–2012: Challenges on the path towards elimination. Malar. J. 2014, 13, 241. [Google Scholar] [CrossRef]
- Department of Health Services. Annual Report 2068/69 (2011/2012) Kathmandu: Department of Health Services, Ministry of Health and Population, Government of Nepal, Kathmandu; Department of Health Services: Kathmandu, Nepal, 2013. Available online: http://www.nnfsp.gov.np/PortalContent.aspx?Doctype=Resources&ID=228 (accessed on 5 September 2018).
- Malla, S.; Thakur, G.D.; Shrestha, S.K.; Banjeree, M.K.; Thapa, L.B.; Gongal, G.; Ghimire, P.; Upadhyay, B.P.; Gautam, P.; Khanal, S.; et al. Identification of all dengue serotypes in Nepal. Emerg. Infect. Dis. 2008, 14, 1669–1670. [Google Scholar] [CrossRef]
- Pandey, B.D.; Nabeshima, T.; Pandey, K.; Rajendra, S.P.; Shah, Y.; Adhikari, B.R.; Gupta, G.; Gautam, I.; Tun, M.M.; Uchida, R.; et al. First isolation of dengue virus from the 2010 epidemic in Nepal. Trop. Med. Health 2013, 41, 103–111. [Google Scholar] [CrossRef]
- Dhimal, M.; Gautam, I.; Kress, A.; Muller, R.; Kuch, U. Spatio-temporal distribution of dengue and lymphatic filariasis vectors along an altitudinal transect in Central Nepal. PLoS Negl. Trop. Dis. 2014, 8, e3035. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, R.; Wells, K.; O’Hara, R.B.; Kalko, E.K.; Renner, S.C. Variable strength of forest stand attributes and weather conditions on the questing activity of Ixodes ricinus ticks over years in managed forests. PLoS ONE 2013, 8, e55365. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Worldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasites Vectors 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, Y.; Kozlova, T.; Kozlovskaya, L. Observations on changes in abundance of questing Ixodes ricinus, castor bean tick, over a 35-year period in the eastern part of its range (Russia, Tula region). Med. Vet. Entomol. 2015, 29, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Challenges in predicting climate and environmental affects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2010, 213, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Hemming, D.; Agusto, F.; Evans, K.J.; Fefferman, N.; Gaff, H.; Gumel, A.; LaDeau, S.; et al. Climate, environmental and socio-economic change: Weighing up the balance in vector-borne disease transmission. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Bayoh, M.N.; Lindsay, S.W. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med. Vet. Entomol. 2004, 18, 174–179. [Google Scholar] [CrossRef]
- Christiansen-Jucht, C.; Parham, P.E.; Saddler, A.; Koella, J.C.; Basáñez, M.G. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasites Vectors 2014, 7, 489. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Imbahale, S.S.; Thomas, M.B.; Takken, W. Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malar. J. 2010, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Monaghan, A.J.; Lozano-Fuentes, S.; Steinhoff, D.F.; Hayden, M.H.; Bieringer, P.E. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J. Med. Entomol. 2014, 51, 496–516. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, M.S. Aedes aegypti the yellow fever mosquito, its life history, bionomics and structure. J. Natl. Med. Assoc. 1962, 54, 132. [Google Scholar]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The Effect of Temperature on Anopheles Mosquito Population Dynamics and the Potential for Malaria Transmission. PLoS ONE 2013, 8, e79276. [Google Scholar] [CrossRef] [PubMed]
- Hoshen, M.B.; Morse, A.P. A weather-driven model of malaria transmission. Malar. J. 2004, 3, 32. [Google Scholar] [CrossRef]
- Zell, R. Global climate change and the emergence/re-emergence of infectious diseases. Int. J. Med. Microbiol. Suppl. 2004, 293, 16–26. [Google Scholar] [CrossRef]
- Epstein, P.R. West Nile virus and the climate. J. Urban Health 2001, 78, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Gage, K.L.; Burkot, T.R.; Eisen, R.J.; Hayes, E.B. Climate and vectorborne diseases. Am. J. Prev. Med. 2008, 35, 436–450. [Google Scholar] [CrossRef]
- Kovats, R.S.; Bouma, M.J.; Hajat, S.; Worrall, E.; Haines, A.E. Niño and health. Lancet 2003, 362, 1481–1489. [Google Scholar] [CrossRef]
- Hamnett, M.P.; Anderson, C.L.; Guard, C.P. The Pacific ENSO Applications Center and the 1997–98 ENSO Warm Event in the US-affiliated Micronesian Islands: Minimizing Impacts Through Rainfall Forecasts and Hazard Mitigation; Pacific ENSO Applications Center: Honolulu, HI, USA, 1999; Available online: http://www.hazards-climate-environment.org/yahoo_site_admin/assets/docs/HCEP_Technical_Reports_Plans_Updated.142163831.pdf (accessed on 4 July 2018).
- Reid, C. Implications of Climate Change on Malaria in Karnataka, India. Thesis (Honors), Brown University, Providence, RI, USA, 2000. [Google Scholar]
- Snow, K.R.; Medlock, J.M. The potential impact of climate change on the distribution and prevalence of mosquitoes in Britain. Eur. Mosq. Bull. 2006, 21, 1–10. [Google Scholar]
- Service, M.W. Ecological and biological studies on Aedes cantans in southern England. J. Applied Ecol. 1977, 14, 159–196. [Google Scholar] [CrossRef]
- Bates, P.A. Leishmania sand fly interaction: Progress and challenges. Curr. Opin. Microbiol. 2008, 11, 340–344. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2007. EFSA J. 2009, 7, 223r. [Google Scholar] [CrossRef]
- Kovats, R.S.; Edvards, S.J.; Charron, D.; Cowden, J.; D’Souza, R.M.; Ebi, K.L.; Gauci, C.; Gerner-Smidt, P.; Hajat, S.; Hales, S.; et al. Climate variability and campylobacter infection: An international study. Int. J. Biometeorol. 2005, 49, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Bannister, L.; Mitchell, G. The ins, outs and round-abouts of malaria. Trends Parasitol. 2003, 19, 209–213. [Google Scholar] [CrossRef]
- Carpenter, S.; Wilson, A.; Barber, J.; Veronesi, E.; Mellor, P.; Venter, G.; Gubbins, S. Temperature dependence of the extrinsic incubation period of Orbiviruses in Culicoides biting midges. PLoS ONE 2011, 6, e27987. [Google Scholar] [CrossRef]
- Danforth, M.E.; Reisen, W.K.; Barker, C.M. Extrinsic incubation rate is not accelerated in recent California strains of West Nile Virus in Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Avenell, D.; Barrass, I.; Leach, S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J. Vector Ecol. 2006, 31, 292–304. [Google Scholar] [CrossRef]
- Jones, K. Campylobacters in water, sewage and the environment. J. Appl. Microbiol. 2001, 90, 68S–79S. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a changing climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Chien, L.C.; Yu, H.L. Impact of meteorological factors on the spatiotemporal patterns of dengue fever incidences. Environ. Int. 2014, 73, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.T.; Higgs, S. Yellow fever: A disease that has yet to be conquered. Annu. Rev. Entomol. 2007, 52, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Adlouni, S.E.; Beaulieu, C.; Ouarda, T.B.M.J.; Gosselin, P.L.; Saint-Hilaire, A. Effects of climate on West Nile Virus transmission risk used for public health decision-making in Quebec. Int. J. Health Geogr. 2007, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Y.; Zhou, S.; Dong, L.; Van Boeckel, T.P.; Cui, Y.J.; Wu, Y.R.; Cazelles, B.; Huang, S.Q.; Yang, R.F.; Grenfell, B.T.; et al. Avian influenza H5N1 viral and bird migration networks in Asia. Proc. Natl. Acad. Sci. USA 2015, 112, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Engelthaler, D.M.; Mosley, D.G.; Cheek, J.E.; Levy, C.E.; Komatsu, K.K.; Ettestad, P.; Davis, T.; Tanda, D.T.; Miller, L.; Frampton, J.W. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerg. Infect. Dis. 1999, 5, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Z.B.; Xu, B. Global spatiotemporal and genetic footprint of the H5N1 avian influenza virus. Int. J. Health Geogr. 2014, 13, 14. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.B.; Jin, Z.Y.; Tan, H.Y.; Xu, B. Risk factors for infectious diseases in backyard poultry farms in the Poyang lake area, China. PLoS ONE 2013, 8, e67366. [Google Scholar] [CrossRef]
- Bai, L.; Woodward, A.; Liu, Q. Temperature and mortality on the roof of the world: A time-series analysis in three Tibetan counties. Sci. Total Environ. 2014, 485, 41–48. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, J.; Jiang, Z.B.; Xu, B. Identifying risk factors of avian infectious disease at household level in Poyang Lake region, China. Prev. Vet. Med. 2014, 116, 151–160. [Google Scholar] [CrossRef]
- Akhtar, R.; Dutt, A.; Wadhwa, V. Health Planning and the Resurgence of Malaria in Urban India. In Urban Health in the Third World; Nangia, S.B., Akhtar, R., Eds.; A.P.H. Publishing Corporation: New Delhi, India, 2002; ISBN 81-7648-293-5. Available online: https://books.google.co.kr/books?id=k8-4cckH9WcC&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 7 September 2018).
- Shah, I.; Deshpande, G.C.; Tardeja, P.N. Outbreak of dengue in Mumbai and predictive markers for dengue shock syndrome. J. Trop. Pediatr. 2004, 50, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.J.; Kovats, R.S.; Armstrong, B.G. Global diarrhoea morbidity, weather and climate. Clim. Res. 2007, 34, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Bissell, R.A. Delayed-impact infectious disease after a natural disaster. J. Emerg. Med. 1983, 1, 59–66. [Google Scholar] [CrossRef]
- Pan American Health Organization. Impact of Hurricane Mitch on Central America. Epidemiol. Bull. 1998, 19, 1–14. Available online: http://www1.paho.org/english/sha/epibul_95- 98/be984mitch.htm (accessed on 7 September 2018).
- Kouadio, I.K.; Aljunid, S.; Kamigaki, T.; Hammad, K.; Oshitani, H. Infectious Diseases Following Natural Disasters: Prevention and Control Measures. Expert Rev. Anti-Infect. Ther. 2012, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Abdelwhab, E.; Selim, A.; Arafa, A.; Galal, S.; Kilany, W.; Hassan, M.; Aly, M.; Hafez, M. Circulation of avian influenza H5N1 in live bird markets in Egypt. Avian Dis. 2010, 54, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Rowling, M. Africa Faces Sharp Rise in Climate Adaptation Costs, UNEP; Thomson Reuters Foundation: London, UK, 2013; Available online: http://news.trust.org//item/20131119134543-8whnr/?source=hptop (accessed on 18 September 2018).
- Hess, J.J.; Mcdowell, J.Z.; Luber, G. Integrating climate change adaptation into public health practice: Using adaptive management to increase adaptive capacity and build resilience. Environ. Health Perspect. 2012, 120, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.E.; Biesbroek, R.; Berrang-Ford, L.; Ford, J.D.; Parker, S.; Fleury, M.D. Public health adaptation to climate change in OECD countries. Int. J. Environ. Res. Public Health 2016, 13, 889. [Google Scholar] [CrossRef]
- Thomson, M.C.; Doblas-Reyes, F.J.; Mason, S.J.; Hagedorn, R.; Connor, S.J.; Phindela, T.; Morse, A.P.; Palmer, T.N. Malaria early warnings based on seasonal climate forecasts from multi-model ensembles. Nature 2006, 439, 576–579. [Google Scholar] [CrossRef]
- Rasul, G. Food, water: And energy security in South Asia: A nexus perspective from the Hindu Kush Himalayan region. Environ. Sci. Policy 2014, 39, 35–48. [Google Scholar] [CrossRef]
- Di Gregorio, M.; Nurrochmat, D.R.; Paavola, J.; Sari, I.M.; Fatorelli, L.; Pramova, E.; Locatelli, B.; Brockhaus, M.; Kusumadewi, S.D. Climate policy integration in the land use sector: Mitigation: Adaptation and sustainable development linkages. Environ. Sci. Policy 2017, 67, 35–43. [Google Scholar] [CrossRef]
- Dessai, S.; Hulme, M. Does climate adaptation policy need probabilities? Clim. Policy 2004, 4, 107–128. [Google Scholar] [CrossRef]
- Pahl-Wostl, C. A conceptual framework for analyzing adaptive capacity and multi-level learning processes in resource governance regimes. Glob. Environ. Chang. 2009, 19, 354–365. [Google Scholar] [CrossRef]
- Hasnoot, M.; Kwakkel, J.H.; Walker, W.E.; Ter Maat, J. Dynamic adaptative policy pathways: A method for crafting robust decisions for a deeply uncertain world. Glob. Environ. Chang. 2013, 23, 485–498. [Google Scholar] [CrossRef]
- Termeer, C.J.; Dewulf, A.; Breeman, G.; Stiller, S.J. Governance capabilities for dealing wisely with wicked problems. Adm. Soc. 2015, 47, 680–710. [Google Scholar] [CrossRef]
- Kreft, S.; Eckstein, D.; Junghans, L.; Kerestan, C.; Hagen, U. Global Climate Risk Index 2015; German Watch: Glashutte, Germany, 2014; pp. 3–59. ISBN 978-3-943704-23-5. Available online: https://germanwatch.org/sites/germanwatch.org/files/publication/10333.pdf (accessed on 18 September 2018).
- Smith, N.; Leiserowitz, A. The role of emotion in global warming policy support and opposition. Risk Anal. 2014, 34, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Schuldt, J.P. Compassion for climate change victims and support for mitigation policy. J. Environ. Psychol. 2016, 45, 192–200. [Google Scholar] [CrossRef]
- Guill, C.K.; Shandera, W.X. The effects of Hurricane Mitch on a community in northern Honduras. Prehosp. Disaster Med. 2001, 16, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Needs assessment following Hurricane Georges—Dominican Republic, 1998. Morb. Mortal. Wkly Rep. 1999, 48, 93–95. [Google Scholar] [CrossRef]
- Toole, M.J. Communicable Diseases and Disease Control. In The Public Health Consequences of Disasters; Noji, E., Ed.; Oxford University Press: New York, NY, USA, 1997; ISBN 978-0195095708. [Google Scholar]
- O’reilly, K.; Dhanju, R.; Goel, A. Exploring “the remote” and “the rural”: Open defecation and latrine use in Uttarakhand, India. World Dev. 2017, 93, 193–205. [Google Scholar] [CrossRef]
- The World Health Organization (WHO)/The United Nations Children’s Fund (UNICEF). Progress in Drinking Water and Sanitation; WHO: Geneva, Switzerland, 2014; ISBN 9789241507240. Available online: https://www.unicef.org/publications/files/JMP_report_2014_webEng.pdf (accessed on 18 September 2018).
- Kumar, S.G.; Kar, S.S.; Jain, A. Health and environmental sanitation in India: Issues for prioritizing control strategies. Indian J. Occup. Environ. Med. 2011, 15, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Haines, A.; McMichael, A.J.; Smith, K.R.; Roberts, I.; Woodcock, J.; Markandya, A.; Armstrong, B.G.; Campbell-Lendrum, D.; Dangour, A.D.; Davies, M.; et al. Public health benefits of strategies to reduce greenhouse-gas emissions: Overview and implications for policy makers. Lancet 2009, 374, 2104–2114. [Google Scholar] [CrossRef]
- Patz, J.A.; Campbell-Lendrum, D.; Holloway, T.; Foley, J.A. Impact of regional climate change on human health. Nature 2005, 438, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.R.; Knutti, J.; Arblaster, J.L.; Dufresne, T.; Fichefet, P.; Friedlingstein, X.; Gao, W.J.; Gutowski, T.; Johns, G.; Krinner, M.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. ISBN 9781107057999. [Google Scholar]
- Smith, K.R.; Woodward, A.; Campbell-Lendrum, D.; Chadee, D.; Honda, Y.; Qiyong Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R. Human Health: Impacts, Adaptation, and Co-Benefits. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; Available online: https://www.ipcc.ch/pdf/assessment-report/ar5/syr/SYR_AR5_FINAL_full_wcover.pdf (accessed on 19 September 2018).
- Luber, G.; Knowlton, K.; Balbus, J.; Frumkin, H.; Hayden, M.; Hess, J.; McGreehin, M.; Sheats, N.; Backer, L.; Beard, C.B.; et al. Human Health. Climate Change Impacts in the United States: The Third National Climate Assessment; Melillo, J.M., Terese, T.C., Yohe, G.W., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2014; pp. 220–256. Available online: https://digital.library.yorku.ca/yul-1120588/chapter-9-human-health-climate-change-impacts-united-states-third-national-climate (accessed on 19 September 2018). [CrossRef]

| Extreme Weather Events | Main Outcomes | References |
|---|---|---|
| El Nino | Found responsible for the outbreak of Malaria in Peru, Bolivia and Ecuador due to extreme rainfall in 1983. The effect of El Nino varied by region. San Francisco and Los Angeles evidenced 30–50% rise in hospitalization with a decrease of 5 °C in minimum average temperature due to influenza epidemics. However, 25–40% hospitalizations were observed in Sacramento with just 5 °C in maximum average temperature. | [30] [56] |
| La Nina | La Nina events cause outbreaks of Japanese encephalitis and West Nile Virus. Occurrence of drought due to the La Nina event was linked to the chikungunya fever epidemic | [30] [57] |
| Quasi-Biennial Oscillation (OBQ) | OBQ was confirmed as the main cause for the emergence of Ross River virus in Queensland. | [58] |
| Drought | Among various categories of drought, agricultural drought is the most severe, and many farmers suffer from mental disorders due to significant losses in crop production. Many end up committing suicide. | [59] |
| Heat Waves | A positive relationship was observed between hospital admission, excessive heat and humidity due to the emergence of angina pectoris. On an annual basis, more than 400 children in the United States die of heatwave-related sicknesses such as diarrhea, typhoid and jaundice. Recent studies provide reliable evidence on the direct relationship between heatwaves and myocardial infarction with congestive heart failure. | [42] [47] [48,49] |
| Flood | A direct association was observed between gastroenteritis and intensity of flood at Lewes in Southern England. | [60] |
| Hurricane | Honduras and Venezuela evidenced malaria and dengue outbreak post a hurricane event. | [6] |
| Cyclone | Cyclone events remarkably increase leptospirosis and cholera incidences. | [52,53] |
| Climate Impacted Variables | Emerged Diseases | Main Outcomes | References |
|---|---|---|---|
| Pathogens | |||
| Japanese Encephalitis Virus (JEV) | Japanese encephalitis viral disease | A temperature range of 25–26 °C is best for transmission of JEV through mosquitoes. | [64,66] |
| Chikungunya Virus (ChikV) | Chikungunya viral fever | Mild winters, summers times with temperature around 20 °C and average rainfall (>50 mm) is optimum for ChickV transmission. | [120] |
| Campylobacter spp. | Campylobacteriosis | Lower surface water temperatures are more favorable for their growth. At higher water temperatures and more intense UV radiations, other bacteria can out-compete them, which can lead to extinction of Campylobacter spp. | [121] |
| Influenza Virus H5N1 | Bird flu/Avian Influenza | H1N1 influenza virus concentration was found to be significantly higher during Asian dust storms compared to other days. | [80] |
| Influenza A virus H1N1 | Influenza (flu) | Temperature and humidity enhance the transmission of influenza A virus (H1N1). | [77] |
| Dengue virus | Dengue | The impact of relative humidity and rainy season over dengue virus propagation were found to be responsible for the outbreak of dengue in Yangon and Singapore. | [79] |
| Leishmania spp | Leishmaniasis | Temperature greatly influences diapause and maturation of L. infantum in infected sandfly. | [114] |
| Vectors | |||
| Plasmodium falciparum (Mosquito) | Malaria | P. falciparum grows at a faster rate in warmer temperatures i.e., it takes 26 days for incubation at 20 °C but only 13 days at 25 °C. | [7] |
| Anopheles fluviatilis; An. dravidicus; An. willmori; An. minimus; An. pseudowillmori; An. maculatus; and An. annularis | Malaria | As a consequence of the rising rate of warming, significant spatiotemporal distribution of vectors was observed in Nepal. Malaria, which was earlier confined to forests near Tarai lowlands (in 38 districts), has expanded to further 68 districts. | [86,87,88] |
| Culex tritaeniorhynchushas | Japanese encephalitis | Rising rate in warming forces Culex to find refuge and hence has expanded from the northern part of India and became endemic in Nepal. | [13] |
| Aedes aegypti | Dengue | The continuous rise in temperature and changes in precipitation pattern accelerates the growth of A. aegypti. | [122] |
| Precipitation can modulate the size, behavior and population of A. aegypti. With rainfall (>50 mm), significant cases of dengue were seen, whereas a temporary decrease in cases was seen during extreme rainfall. | [123] | ||
| A significant spatiotemporal distribution of mosquitoes was observed over a wide area due to rise in warming rate. | [89,90,91] | ||
| The growth of A. aegypti ceases when water temperature surpasses 34 °C and for the adult one if the temperature exceeds 40 °C. | [103] | ||
| Aedes spp | Malaria, Dengue, Chikungunya | Effect of temperature on Aedes species activities: Larvae development: 38 days at 8 °C and 18 days at 12 °C. Bloodmeal digestion: 30 days at 4 °C and 5 days at 20 °C. Embryonic developments: 42 days at 4 °C, 22 days at 12 °C and 8 days at 20 °C. | [112,113] |
| Aedes spp.; Haemagogus spp.; Sabethes spp. (Mosquito) | Yellow Fever | Warmer climate encourages the growth of mosquito species, resulting in outbreaks of yellow fever in Africa and South America. | [124] |
| Culex spp. (Mosquito) | West Nile Fever | The aggressiveness of Culex spp. was found to be strongly correlated with humidity, rainfall and temperature changes. | [125] |
| Ixodes Ricinus (Tick) | Tick-borne encephalitis | Faster development and increased activity with rise in humidity and temperature. | [20] |
| Due to mild winters and extended spring and autumn, I. ricinus coverage area increases from 12.5% to around 26.8% in Sweden during 1990–2008. | [94] | ||
| Oncomelania hupensis (Snail) | Schistosomiasis | With continuous rise in winter temperature, O. hupensis widens its distribution range, thereby spreading schistosomiasis in northern China. | [12] |
| Transmission | |||
| Influenza Virus H5N1 | Bird flu/Avian Influenza | The H5N1 outbreak is associated with wild fowl migration. | [126] |
| H5N1 viruses were found to be carried away to distant areas during Asian dust storms. | [80] | ||
| Hantavirus | Hantavirus pulmonary Syndrome | During extreme conditions like floods, deer mice may approach human dwellings in search of food and can transmit disease. | [127] |
| Leishmania infantum | Leishmaniasis | The biting activity of sand fly is found to be more common in summer months, although too hot and dry conditions are not suitable for survival of sand flies. | [114] |
| Campylobacter spp. | Campylobacteriosis | Colonization of campylobacter along with those meats have an exponential relationship with rising temperature. | [115,116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.D.; Thi Vu, H.H.; Lai, Q.T.; Ahn, J.W. Aggravation of Human Diseases and Climate Change Nexus. Int. J. Environ. Res. Public Health 2019, 16, 2799. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152799
Khan MD, Thi Vu HH, Lai QT, Ahn JW. Aggravation of Human Diseases and Climate Change Nexus. International Journal of Environmental Research and Public Health. 2019; 16(15):2799. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152799
Chicago/Turabian StyleKhan, Mohd Danish, Hong Ha Thi Vu, Quang Tuan Lai, and Ji Whan Ahn. 2019. "Aggravation of Human Diseases and Climate Change Nexus" International Journal of Environmental Research and Public Health 16, no. 15: 2799. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152799