Shift in Potential Malaria Transmission Areas in India, Using the Fuzzy-Based Climate Suitability Malaria Transmission (FCSMT) Model under Changing Climatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Malaria Transmission in India
2.2. Climate Model Data
2.3. Selection of Model Indices
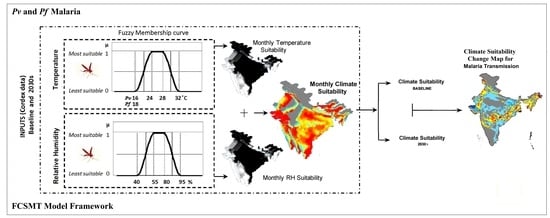
2.4. Fuzzy-Based Climate Suitability Malaria Transmission (FCSMT) Model Framework
3. Results
3.1. Monthly Climate Suitability for Baseline and Projected 2030s for Pv and Pf
3.2. Composite Climate Suitability Map for Baseline and Projected 2030s for Pv and Pf
3.3. Changes in Climate Suitability between Baseline and Projected 2030s
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Vector Borne Disease Control Programme (NVBDCP). National Strategy Plan Malaria Elimination in India 2017–2022, Ministry of Health and Family Welfare, GoI. 2017. Available online: http://www.indiaenvironmentportal.org.in/files/file/nsp_2017-2022-updated.pdf (accessed on 5 December 2017).
- Gill, C.A. The role of meteorology and malaria. Indian J. Med. Res. 1921, 8, 633–693. [Google Scholar]
- Craig, M.H.; Snow, R.W.; le Sueur, D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today 1999, 15, 105–111. [Google Scholar] [CrossRef]
- Grover-Kopec, E.K.; Blumenthal, M.B.; Ceccato, P.; Dinku, T.; Omumbo, J.A.; Connor, S.J. Web-based climate information resources for malaria control in Africa. Malar. J. 2006, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.C.; Pahwa, S.; Dash, A.P. Climate change and malaria in India: Interplay between temperatures and mosquitoes. Reg. Health Forum 2008, 12, 27–31. [Google Scholar]
- Dhiman, R.C.; Pahwa, S.; Dhillon, G.P.S.; Dash, A.P. Climate change and threat of vector-borne diseases in India: Are we prepared? Parasitol. Res. 2010, 106, 763–773. [Google Scholar] [CrossRef]
- Bhadra, A.; Ionides, L.L.; Laneri, K.; Pascual, M.; Bouma, M.; Dhiman, R.C. Malaria in Northwest India: Data analysis via partially observed stochastic differential equation models driven by Lévy noise. J. Am. Stat. Assoc. 2011, 106, 440–451. [Google Scholar] [CrossRef]
- Blanford, J.I.; Blanford, S.; Crane, R.G.; Mann, M.E.; Paaijmans, P.; Schreiber, K.V.; Thomas, M.B. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 2013, 3, 1300. [Google Scholar] [CrossRef]
- Cash, B.A.; Rodó, X.; Ballester, J.; Bouma, M.J.; Baeza, A.; Dhiman, R.; Pascual, R. Malaria epidemics and the influence of the tropical South Atlantic on the Indian monsoon. Nat. Clim. Chang. 2013, 3, 502–507. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M. Global land moisture trends: Drier in dry and wetter in wet over land. Sci. Rep. 2015, 5, 18018. [Google Scholar] [CrossRef]
- Jetten, T.H.; Martens, W.J.; Takken, W. Model stimulations to estimate malaria risk under climate change. J. Med. Entomol. 1996, 33, 361–371. [Google Scholar] [CrossRef]
- Rogers, D.J. Changes in disease vector distributions. In Climate Change and Southern Africa: An Exploration of Some Potential Impacts and Implications in the SADC Region; Hulme, M., Ed.; Climate Research Unit; University of East Anglia: Norwich, UK, 1996; pp. 49–55. [Google Scholar]
- Sutherst, R.W. Implications of global change and climate variability for vector-borne diseases: Generic approaches to impact assessments. Int. J. Parasitol. 1998, 28, 935–945. [Google Scholar] [CrossRef]
- Zhou, G.; Minakawa, N.; Githeko, A.K.; Yan, G. Climate variability and malaria epidemics in the highlands of East Africa. Trends Parasitol. 2005, 21, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Ahumada, J.A.; Chaves, L.F.; Rodó, X.; Bouma, M. Malaria resurgence in the East African highlands: Temperature trends revisited. Proc. Natl. Acad. Sci. USA 2006, 103, 5829–5834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanescu, L.; Bodale, I.; Florescu, S.-A.; Roman, C.; Acatrinei, D.; Miron, L. Climate change is increasing the risk of the reemergence of malaria in Romania. BioMed Res. Int. 2016, 8560519. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.C.; Sternberg, E.D.; Thomas, M.B. Malaria transmission potential could be reduced with current and future climate change. Sci. Rep. 2016, 6, 27771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouma, M.J.; Baeza, A.; terVeen, A.; Pascual, M. Global malaria maps and climate change: A focus on East African highlands. Trends Parasitol. 2011, 27, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Caminadea, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of climate change on global malaria distribution. Proc. Natl. Acad. Sci. USA 2014, 111, 3286–3291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauderdale, J.M.; Caminade, C.; Heath, A.E.; Jones, A.E.; MacLeod, D.A.; Gouda, K.C.; Murty, U.S.; Goswami, P.; Mutheneni, S.R.; Morse, A.P. Towards seasonal forecasting of malaria in India. Malar. J. 2014, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, S.M.; Mutheneni, S.R.; Chenna, S.; Parasaram, V.; Kadiri, M.R. Climate drivers on malaria transmission in Arunachal Pradesh, India. PLoS ONE 2015, 10, e0119514. [Google Scholar] [CrossRef]
- Dhiman, R.C.; Chavan, L.; Pant, M.; Pahwa, S. National and regional impacts of climate change on malaria by 2030. Curr. Sci. 2011, 101, 372–383. [Google Scholar]
- MARA/ARMA. Towards an Atlas of Malaria Risk in AFRICA; First Technical Report of the MARA/ARMA Collaboration; MARA/ARMA: Durban, South Africa, 1998; Available online: https://www.mara-database.org/docs/ENG_MARA_Tech_Rep.pdf (accessed on 5 December 2017).
- Snow, R.W.; Gouws, E.; Omumbo, J.; Rapuoda, B.; Craig, M.H.; Tanser, F.C.; le Sueur, D.; Ouma, J. Models to predict the intensity of Plasmodium falciparum transmission: Applications to the burden of disease in Kenya. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 601–606. [Google Scholar] [CrossRef]
- Ebi, K.L.; Hartman, J.; Chan, N.; Mcconnell, J.; Schlesinger, M.; Weyant, J. Climate suitability for stable malaria transmission in Zimbabwe under different climate change scenarios. Clim. Chang. 2005, 73, 375–393. [Google Scholar] [CrossRef]
- Buczak, A.L.; Baugher, B.; Guven, E.; Ramac-Thomas, L.C.; Elbert, Y.; Babin, S.M.; Lewis, S.H. Fuzzy association rule mining and classification for the prediction of malaria in South Korea. BMC Med. Inform. Decis. Mak. 2015, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Directorate of National Vector Borne Disease Control Programme (NVBDCP). National Framework for Malaria Elimination in India 2016–2030; Directorate General of Health Services Ministry of Health and Family Welfare, Govt of India: New Delhi, India, 2016; pp. 1–43.
- Rao, T.R. The Anophelines of India, 2nd ed.; Malaria Research Centre, Indian Council of Medical Research: New Delhi, India, 1984. [Google Scholar]
- Sharma, V.P. Battling malaria iceberg incorporating strategic reforms in achieving millennium development goals & malaria elimination in India. Indian J. Med. Res. 2012, 136, 907–925. [Google Scholar] [PubMed]
- Singh, P.; Lingala, M.A.L.; Sarkar, S.; Dhiman, R.C. Mapping of malaria vectors at district level in India: Changing scenario and identified gaps. Vector Borne Zoonotic Dis. 2017, 17, 91–98. [Google Scholar] [CrossRef] [PubMed]
- National Vector Borne Disease Control Programme (NVBDCP). Malaria Situation in India. Ministry of Health and Family Welfare, Govt. of India, New Delhi, India, 2016. Available online: http://nvbdcp.gov.in/malaria-new.html (accessed on 12 December 2017).
- Rogelj, J.; Meinshausen, M.; Knutti, R. Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nat. Clim. Chang. 2012. [Google Scholar] [CrossRef]
- Taylor, K.E.; Stouffer, R.J.; Meehl, G.A. An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 2012, 93, 485–498. [Google Scholar] [CrossRef]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma ML, T.; Lamarque, J.F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.B.; Riahi, K.; et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213–241. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, R.K.; Joshi, J.; Jayaraman, M.; Bala, G.; Ravindranath, N.H. Multi-model climate change projections for India under representative concentration pathways. Curr. Sci. 2012, 103, 1–12. [Google Scholar]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Arias, S.D.; Lamberty, B.B.; Wise, M.A.; Clarke, L.E.; et al. RCP 4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Laneri, K.; Bhadra, A.; Ionides, L.L.; Bouma, M.; Dhiman, R.C.; Rajpal, S.Y.; Pascual, M. Forcing versus feedback: Epidemic malaria and monsoon rains in northwest India. PLoS Comput. Biol. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bouma, M.; Dhiman, R.C.; Pascual, M. Predictability of epidemic malaria under non-stationary conditions with process-based models combining epidemiological updates and climate variability. Malar. J. 2015, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.A. The Season Periodicity of Malaria; Churchill: London, UK, 1938. [Google Scholar]
- Russel, P.F.; Luther, S.W.; Manwell, R.D. Practical Malariology; Saunders: London, UK, 1946. [Google Scholar]
- Sluiter, R. Interpolation methods for climate data: Literature review. In KNMI Intern Rapport: IR 2009-04 Version 1.0; R&D Information and Observation Technology: De Bilt, The Netherlands, 2009. [Google Scholar]
- Fisher, P.; Unwin, D.J. Representing geographical information system. In Representing GIS; Fisher, P., Unwin, D.J., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005; pp. 1–13. [Google Scholar]
- Zadeh, L.A. Fuzzy Sets. Inform. Control 1965, 8, 338–353. [Google Scholar] [CrossRef]
- Martens, W.J.M.; Niessen, L.W.; Rotmans, J.; Mcmichael, A.J. Potential impacts of global climate change on malaria risk. Environ. Health Perspect. 1995, 103, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Krijn, P.P.; Leah, R.J. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 2013, 16, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sharma, C.; Dhiman, R.C.; Mitra, A.P. Climate change and malaria in India. Curr. Sci. India 2006, 90, 369–375. [Google Scholar]
- Bayoh, M.N. Studies on the Development and Survival of Anopheles Gambiae Sensu Stricto at Various Temperatures and Relative Humidities. Ph.D. Thesis, University of Durham, Durham, UK, 2001. [Google Scholar]
- Yamana, T.K.; Eltahir, E.A.B. Incorporating the effects of humidity in a mechanistic model of Anopheles gambiae mosquito population dynamics in the Sahel region of Africa. Parasites Vectors 2013, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmayer, W.L. The measurement of adult mosquito population changes-some considerations. J. Am. Mosq. Control Assoc. 1985, 1, 328–350. [Google Scholar]
- Rudolfs, W. Observations on the relations between atmospheric conditions and the behavior of mosquitoes. Bull. N. J. Agric. Exp. Stn. 1923, 388, 32. [Google Scholar]
- Rudolfs, W. Relation between temperature, humidity and activity of house mosquitoes. J. N. Y. Entomol. Soc. 1925, 33, 163–169. [Google Scholar]
- Platt, R.; Love, G.J.; Williams, E.L. A positive response between relative humidity and the distribution and abundance of Aedes vexans. Ecology 1958, 39, 167–169. [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109, 141–161. [Google Scholar] [PubMed]
- Lindsay, S.W.; Birley, M.H. Climate change and malaria transmission. Ann. Trop. Med. Parasitol. 1996, 90, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Cox, J.; Rogers, D.J.; Randolph, S.E.; Stern, D.I.; Shanks, G.D.; Myers, M.; Snow, R.W. Climate change and the resurgence malaria in the East African highlands. Nature 2002, 415, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Xiong, H.Y.; Yi, D.; Chai, G.J.; Yang, X.W.; Liu, L. Study on meteorological factors-based neural network model of malaria. Zhonghua Liu Xing Bing Xue Za Zhi 2003, 24, 831–834. [Google Scholar] [PubMed]







| Parameters | Temperature (in °C) | RH (in %) | Remarks | |
|---|---|---|---|---|
| Pv | a | 16 | 40 | Least suitable |
| b | 24 | 55 | Most suitable | |
| c | 28 | 80 | Most suitable | |
| d | 32 | 95 | Least suitable | |
| Pf | a | 18 | 40 | Least suitable |
| b | 24 | 55 | Most suitable | |
| c | 28 | 80 | Most suitable | |
| d | 32 | 95 | Least suitable |
| States | Sl. No. | Districts | Circles/Tehsils | No. of Months | Projected Months |
|---|---|---|---|---|---|
| Jammu and Kashmir | 1 | Pulwama | Chrar-e-Shrief | up to 1 | July |
| 2 | Srinagar | Shrinagar South | up to 2 | July, August | |
| 3 | Baramula | Rafaibad, Baramula, Kheeri, Boniar, Tangmarg | up to 2 | July, August | |
| 4 | Bagdam | All tehsils | up to 2 | July, August | |
| 5 | Anantnag | Dooru, Kokemag | up to 2 | July, August | |
| 6 | Shupiyan | All tehsils | up to 2 | July, August | |
| 7 | Kulgam | All tehsils | up to 2 | July, August | |
| 8 | Ganderbal | Ganderbal | up to 2 | July, August | |
| 9 | Ramban | Banihal | up to 2 | July, August | |
| Himachal Pradesh | 10 | Chamba | Chamba | up to 2 | July, August |
| Uttarakhand | 11 | Champawat | Lohaghat | up to 2 | June, July |
| 12 | Bageshwar | Kapkot | up to 3 | June, July, August | |
| 13 | Pithoragarh | Berinag, Didihat, Gangolihat, Pithoragarh | up to 4 | June, July, August, September |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; Gangare, V.; Singh, P.; Dhiman, R.C. Shift in Potential Malaria Transmission Areas in India, Using the Fuzzy-Based Climate Suitability Malaria Transmission (FCSMT) Model under Changing Climatic Conditions. Int. J. Environ. Res. Public Health 2019, 16, 3474. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16183474
Sarkar S, Gangare V, Singh P, Dhiman RC. Shift in Potential Malaria Transmission Areas in India, Using the Fuzzy-Based Climate Suitability Malaria Transmission (FCSMT) Model under Changing Climatic Conditions. International Journal of Environmental Research and Public Health. 2019; 16(18):3474. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16183474
Chicago/Turabian StyleSarkar, Soma, Vinay Gangare, Poonam Singh, and Ramesh C. Dhiman. 2019. "Shift in Potential Malaria Transmission Areas in India, Using the Fuzzy-Based Climate Suitability Malaria Transmission (FCSMT) Model under Changing Climatic Conditions" International Journal of Environmental Research and Public Health 16, no. 18: 3474. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16183474