Occupational Class Differences in Long-Term Sickness Absence Due to Breast Cancer during 2005–2013: A Population-Based Study among Finnish Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements of Sickness Absence
2.2. Measurements of Occupational Class
2.3. Statistical Methods
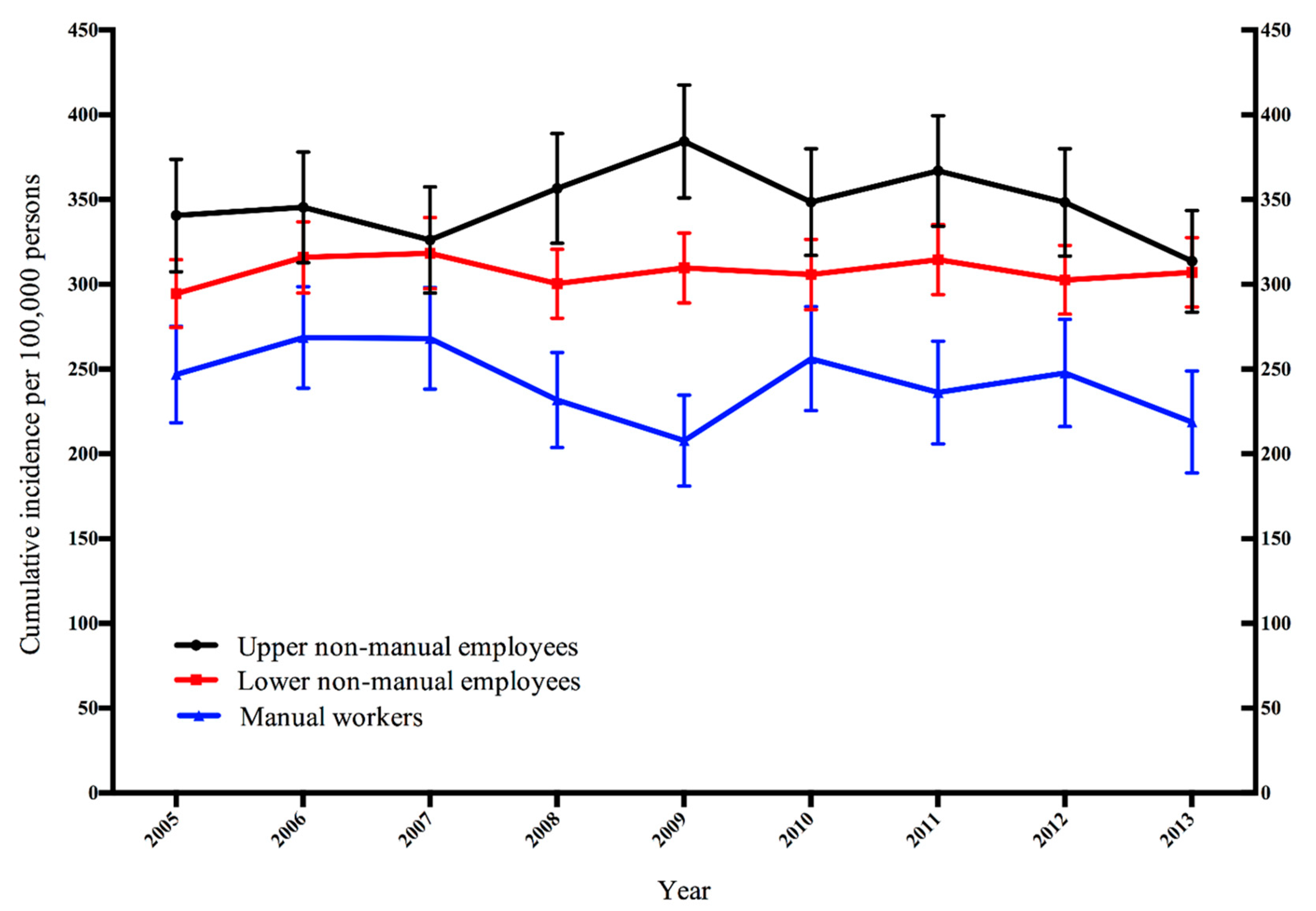
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| ICD-10 | The International Classification of Diseases, 10th revision |
| Kela | The Social Insurance Institution of Finland |
| WHO | World Health Organization |
References
- Mackenbach, J.P.; Stirbu, I.; Roskam, A.R.; Schaap, M.M.; Menvielle, G.; Leinsalu, M.; Kunst, A.E. Socioeconomic Inequalities in Health in 22 European Countries. N. Engl. J. Med. 2008, 358, 2468–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenbach, J.P.; Kulhánová, I.; Artnik, B.; Bopp, M.; Borrell, C.; Clemens, T.; Costa, G.; Dibben, C.; Kalediene, R.; Lundberg, O.; et al. Changes in mortality inequalities over two decades: Register based study of European countries. BMJ 2016, 353, i1732. [Google Scholar] [CrossRef] [PubMed]
- Danø, H.; Hansen, K.; Jensen, P.; Petersen, J.; Jacobsen, R.; Ewertz, M.; Lynge, E. Fertility pattern does not explain social gradient in breast cancer in denmark. Int. J. Cancer 2004, 111, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe-a systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Pukkala, E.; Martinsen, J.; Lynge, E.; Gunnarsdottir, H.; Sparn, P.; Tryggvadottir, L.; Weiderpass, E.; Kjaerheim, K. Occupation and cancer follow-up of 15 million people in five Nordic countries. Acta. Oncol. 2009, 48, 646–790. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease StudyGlobal Burden of Cancer 2015Global Burden of Cancer 2015. Oncology 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Łyszczarz, B.; Nojszewska, E. Productivity losses and public finance burden attributable to breast cancer in Poland, 2010–2014. BMC Cancer 2017, 17, 676. [Google Scholar] [CrossRef] [PubMed]
- Karim-Kos, H.; de Vries, E.; Soerjomataram, I.; Lemmens, V.; Siesling, S.; Coebergh, J. Recent trends of cancer in Europe: A combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur. J. Cancer 2008, 44, 1345–1389. [Google Scholar] [CrossRef]
- Finnish Cancer Registry. (Rinta C50) Uudet Syöpätapaukset 2016, Ikävakioitu Maailman Vakioväestöllä, Suhde Per 100,000. 2019. Available online: https://syoparekisteri.fi/tilastot/tautitilastot (accessed on 1 July 2019).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Association of the Nordic Cancer Registries (Rinta) Ikävakioitu Suhteellinen 5-Vuotiselossaolo-Osuus. Available online: http://www-dep.iarc.fr/NORDCAN/FI/StatsFact.asp?cancer=200&country=246 (accessed on 1 July 2019).
- Vehko, T.; Arffman, M.; Manderbacka, K.; Pukkala, E.; Keskimäki, I. Differences in mortality among women with breast cancer by income—A register-based study in Finland. Scand. J. Public Health 2016, 44, 630–637. [Google Scholar] [CrossRef]
- Drolet, M.; Maunsell, E.; Mondor, M.; Brisson, C.; Brisson, J.; Mâsse, B.; Deschênes, L. Work absence after breast cancer diagnosis: A population-based study. CMAJ 2005, 173, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Kvillemo, P.; Mittendorfer-Rutz, E.; Bränström, R.; Nilsson, K.; Alexanderson, K. Sickness Absence and Disability Pension After Breast Cancer Diagnosis: A 5-Year Nationwide Cohort Study. J. Clin. Oncol. 2017, 35, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Alexanderson, K.; Norlund, A. Swedish Council on Technology Assessment in Health Care (SBU). Chapter 1. Aim, background, key concepts, regulations, and current statistics. Scan. J. Public Health 2004, 32, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Bouknight, R.; Bradley, C.; Luo, Z. Correlates of return to work for breast cancer survivors. J. Clin. Oncol. 2006, 24, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, M.; Piha, K.; Rahkonen, O.; Martikainen, P.; Lahelma, E. Explaining occupational class differences in sickness absence: Results from middle-aged municipal employees. J. Epidemiol. Community Health 2010, 64, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, J.; Blomgren, J.; Pietiläinen, O.; Lahelma, E.; Rahkonen, O. Occupational class differences in long sickness absence: A register-based study of 2.1 million Finnish women and men in 1996–2013. BMJ 2017, 7, e014325. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A. Breast cancer in young women. Nat. Rev. Clin. Oncol. 2012, 9, 460–470. [Google Scholar] [CrossRef] [Green Version]
- Statistics Finland Classification of Socio-Economic Groups. 1989. Available online: https://www.stat.fi/meta/luokitukset/sosioekon_asema/001-1989/index_en.html (accessed on 1 July 2019).
- Finnish Advisory Board on Research Integrity Ethical Review in Human Sciences. Available online: http://www.tenk.fi/en/ethical-review-in-human-sciences (accessed on 1 July 2019).
- Hensing, G.; Alexanderson, K.; Allebeck, P.; Bjurulf, P. How to measure sickness absence? Literature review and suggestion of five basic measures. Scand. J. Soc. Med. 1998, 26, 133–144. [Google Scholar] [CrossRef]
- Borg, K.; Goine, H.; Söderberg, E.; Marnetoft, S.; Alexanderson, K. Comparison of seven measures of sickness absence based on data from three counties in Sweden. Work 2006, 26, 421–428. [Google Scholar]
- Hensing, G. The measurements of sickness absence—A theoretical perspective. Nor. Epidemiol. 2009, 19, 147–151. [Google Scholar] [CrossRef]
- McPherson, K.; Steel, C.; Dixon, J. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ 2000, 321, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Nisén, J.; Myrskylä, M.; Silventoinen, K.; Martikainen, P. Effect of family background on the educational gradient in lifetime fertility of Finnish women born 1940–1950. Popul. Stud. 2014, 68. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.; Jackson, C.A.; Rowlands, J.V.; Mishra, G.D. Socioeconomic position, lifestyle factors and age at natural menopause: A systematic review and meta-analyses of studies across six continents. Int. J. Epidemiol. 2014, 43, 1542–1562. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, P.; Penninkilampi, R.; Edirimanne, S.; Eslick, G. Physical Activity and Weight Loss Reduce the Risk of Breast Cancer: A Meta-analysis of 139 Prospective and Retrospective Studies. Clin. Breast Cancer 2018, 18, 601–612. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation (WHO Technical Report Series 894); WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Kullberg, C.; Selander, J.; Albin, M.; Borgquist, S.; Manjer, J.; Gustavsson, P. Female white-collar workers remain at higher risk of breast cancer after adjustments for individual risk factors related to reproduction and lifestyle. Occup. Environ. Med. 2017, 74, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Pudrovska, T.; Carr, D.; McFarland, M.; Collins, C. Higher-status occupations and breast cancer: A life-course stress approach. Soc. Sci. Med. 2013, 89, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Kjellen, M.; von Euler-Chelpin, M. Socioeconomic status as determinant for participation in mammography screening: Assessing the difference between using women’s own versus their partner’s. Int. J. Public Health 2010, 55, 209–215. [Google Scholar] [CrossRef]
- Marmot, M.G.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The benefits and harms of breast cancer screening: An independent review. Br. J. Cancer 2013, 108, 2205–2240. [Google Scholar] [CrossRef]
- Statistics Finland Labour Market. Available online: http://tilastokeskus.fi/tup/suoluk/suoluk_tyoelama_en.html (accessed on 1 July 2019).
- Johnsson, A.; Fornander, T.; Rutqvist, L.E.; Olsson, M. Work status and life changes in the first year after breast cancer diagnosis. Work 2011, 38, 337–346. [Google Scholar]
- Plym, A.; Bower, H.; Fredriksson, I.; Holmberg, L.; Lambert, P.; Lambe, M. Loss in working years after a breast cancer diagnosis. Br. J. Cancer 2018, 118, 738. [Google Scholar] [CrossRef]
- Lahelma, E.; Martikainen, P.; Laaksonen, M.; Aittomäki, A. Pathways between socioeconomic determinants of health. J. Epidemiol. Community Health 2004, 58, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuser, C.; Halbach, S.; Kowalski, C.; Enders, A.; Pfaff, H.; Ernstmann, N. Sociodemographic and disease-related determinants of return to work among women with breast cancer: A German longitudinal cohort study. BMC Health Serv. Res. 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.S.; Overgaard, C.; Boggild, H.; Garne, J.P.; Lund, T.; Overvad, K.; Fonager, K. The long-term financial consequences of breast cancer: A Danish registry-based cohort study. BMC Public Health 2017, 17. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: A licence allowing the use data of Kela and Statistics Finland was granted for the present study, Kela (permission number 59/522/2015) and Statistics Finland (permission number TK-53-1106-15). The data are not publicly available but a licence can be requested from the data providers. |

| Occupational Class | Year | |||
|---|---|---|---|---|
| 2005 | 2009 | 2013 | ||
| Upper non-manual | Population at risk, N (%) | 111,016 (22) | 125,543 (24) | 128,783 (25) |
| Number of SA episodes | 354 | 456 | 389 | |
| Lower non-manual | Population at risk, N (%) | 270,150 (54) | 279,000 (54) | 286,411 (56) |
| Number of SA episodes | 766 | 869 | 894 | |
| Manual workers | Population at risk, N (%) | 118,612 (24) | 114,775 (22) | 97,953 (19) |
| Number of SA episodes | 296 | 247 | 277 | |
| Total | Population at risk, N (%) | 499,778 (100) | 519,318 (100) | 513,147 (100) |
| Number of SA episodes | 1416 | 1572 | 1560 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suur-Uski, J.; Pekkala, J.; Blomgren, J.; Pietiläinen, O.; Rahkonen, O.; Mänty, M. Occupational Class Differences in Long-Term Sickness Absence Due to Breast Cancer during 2005–2013: A Population-Based Study among Finnish Women. Int. J. Environ. Res. Public Health 2019, 16, 3477. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16183477
Suur-Uski J, Pekkala J, Blomgren J, Pietiläinen O, Rahkonen O, Mänty M. Occupational Class Differences in Long-Term Sickness Absence Due to Breast Cancer during 2005–2013: A Population-Based Study among Finnish Women. International Journal of Environmental Research and Public Health. 2019; 16(18):3477. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16183477
Chicago/Turabian StyleSuur-Uski, Johanna, Johanna Pekkala, Jenni Blomgren, Olli Pietiläinen, Ossi Rahkonen, and Minna Mänty. 2019. "Occupational Class Differences in Long-Term Sickness Absence Due to Breast Cancer during 2005–2013: A Population-Based Study among Finnish Women" International Journal of Environmental Research and Public Health 16, no. 18: 3477. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16183477





