Practical Issues in Early Switching from Intravenous to Oral Antibiotic Therapy in Children with Uncomplicated Acute Hematogenous Osteomyelitis: Results from an Italian Survey
Abstract
:1. Introduction
2. Methods
3. Results
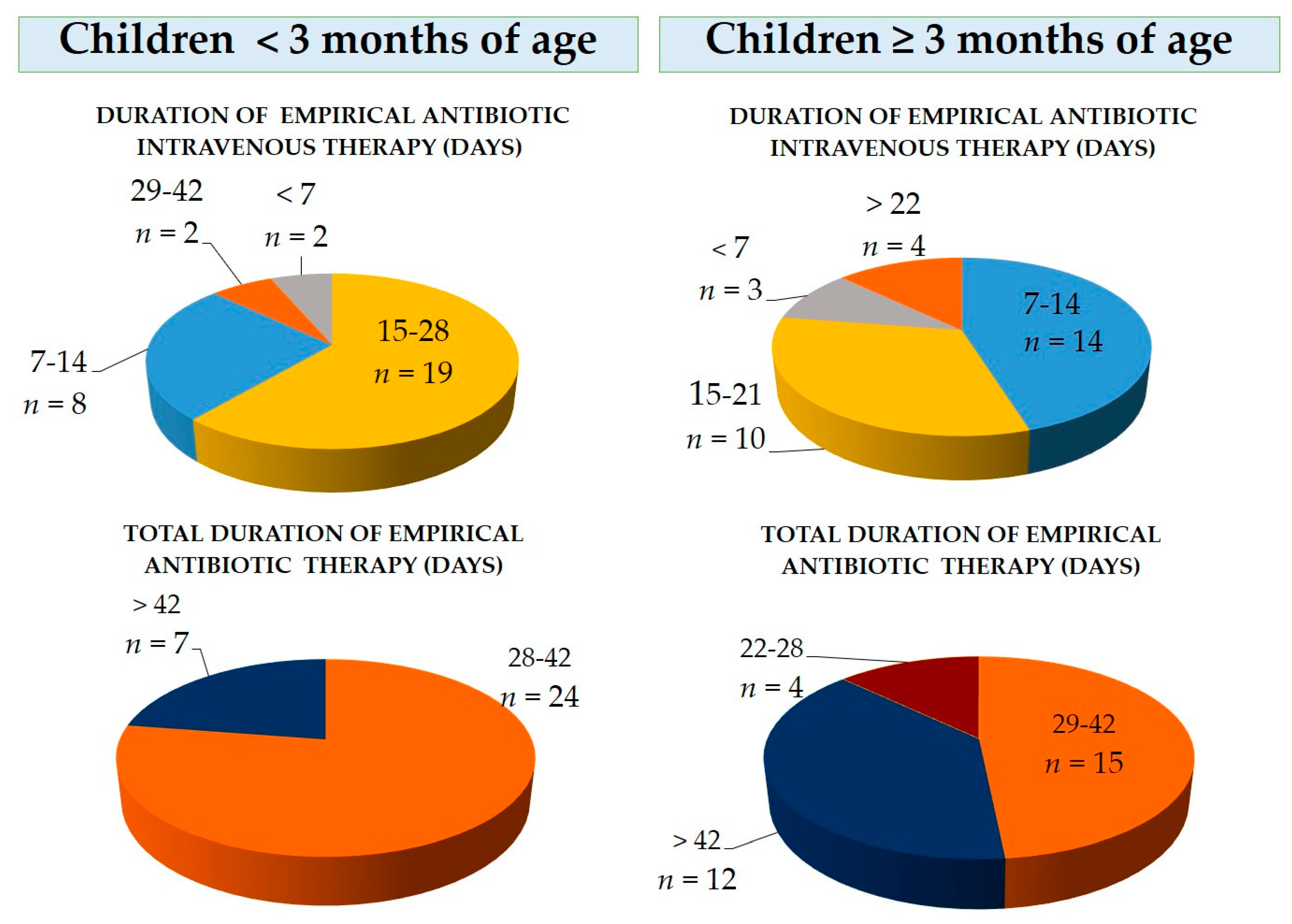
3.1. First-Choice Antibiotic Therapy for Empiric Therapy in Children Aged < 3 Months
3.2. First-Choice Antibiotic Therapy for Empiric Therapy in Children Aged ≥3 Months
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
Questionnaire
- How many children with acute haematogenous osteomyelitis do you manage in your clinic annually?
- Which are the antibiotics used as first choice drugs in empirical therapy in children under 3 months with uncomplicated osteomyelitis?
- When do you switch from IV to PO therapy in children < 3 months of age in absence of complications?
- What are the first choice PO antibiotics used in oral therapy in children aged < 3 months?
- Which is the total duration of treatment (IV+PO) in children aged < 3weeks?
- Which are the antibiotics used as first choice drugs in empirical therapy in children ≥ 3 months with uncomplicated osteomyelitis?
- When do you switch from IV to PO therapy in children ≥ 3 months of age in absence of complications?
- What are the first choice antibiotics used in PO therapy in children aged ≥ 3 months?
- Which is the total duration of antibiotic treatment (IV+PO) in children aged ≥ 3weeks?
- Which factors orient / influence your choice to switch from IV to PO treatment?
References
- Chiappini, E.; Camposampiero, C.; Lazzeri, S.; Indolfi, G.; De Martino, M.; Galli, L. Epidemiology and Management of Acute Haematogenous Osteomyelitis in a Tertiary Paediatric Center. Int. J. Environ. Res. Public Health 2017, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Milcent, K.; Guitton, C.; Koné-Paut, I. French nationwide survey about management of acute osteomyelitis in children. Arch Pediatr. 2009, 16, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Núñez, E.; Camacho, M.; Clemente, D.; Fernández-Cooke, E.; Alcobendas, R.; Mayol, L.; Soler-Palacin, P.; Oscoz, M.; Saavedra-Lozano, J.; et al. Epidemiology and Management of Acute, Uncomplicated Septic Arthritis and Ostemyelitis. Pediatr. Infect. Dis. J. 2016, 35, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.; Falup-Pecurariu, O.; Faust, S.; Girschick, H.; Hartwig, N.; Kaplan, S.; Lorrot, M.; Mantadakis, E.; Peltola, H.; Rojo, P.; et al. Practice Guideline Bone and Join infections. 2017. Available online: http://links.lww.com/INF/C729 (accessed on 11 April 2019).
- Peltola, H.; Pääkkönen, M.; Kallio, P.; Kallio, M.J.; Osteomyelitis-Sptic Arthritis Study Group. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: Prospective, randomized trial on 131 culture-positive cases. Pediatr. Infect. Dis. J. 2010, 29, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Michelow, I.C.; Mandell, J.G. Sequential Intravenous Oral Antibiotic Therapy for Osteomyelitis: How Short Is Long Enough? JAMA Pediatr. 2015, 169, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Jagodzinski, N.A.; Kanwar, R.; Graham, K.; Bache, C.E. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J. Pediatr. Orthop. 2009, 29, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Pääkkönen, M.; Kallio, M.J.; Kallio, P.E.; Peltola, H. Shortened hospital stay for childhood bone and joint infections: Analysis of 265 prospectively collected culture-positive cases in 1983–2005. Scand. J Infect. Dis. 2012, 44, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Hsieh, R.W.; Yen, H.T.; Hsu, T.C.; Chen, C.Y.; Chen, Y.C.; Lee, C.C. Short- versus long-course antibiotics in osteomyelitis: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2019, 53, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Keren, R.; Shah, S.S.; Srivastava, R.; Rangel, S.; Bendel-Stenzel, M.; Harik, N.; Hartley, J.; Lopez, M.; Seguias, L.; Tieder, J.; et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015, 169, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Krzysztofiak, A.; Bozzola, E.; Gabiano, C.; Esposito, S.; Lo Vecchio, A.; Govoni, M.R.; Vallongo, C.; Dodi, I.; Castagnola, E.; et al. Risk factors associated with complications/sequelae of acute and subacute haematogenousosteomyelitis: An Italian multicenter study. Expert Rev. Anti. Infect. Ther. 2018, 16, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Rouse, C.; Mistry, P.; Rayner, O.; Nickless, J.; Wan, M.; Southern, K.W.; Batchelor, H.K. A mixed methods study of the administration of flucloxacillin oral liquid; identifying strategies to overcome administration issues of medicines with poor palatability. Int. J. Pharm. Pract. 2017, 25, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S.N. Prescribing for children - taste and palatability affect adherence to antibiotics: A review. Arch. Dis. Child. 2012, 97, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Grimprel, E.; Lorrot, M.; Haas, H.; Pinquier, D.; Parez, N.; Ferroni, A.; Cohen, R.; Paediatric Infectious Diseases Group of the French Society of Paediatricis (GPIP). Osteoarticular infections: Therapeutic proposals of the Paediatric Infectious Diseases Group of the French Society of Paediatrics (GPIP). Arch. Pediatr. 2008, 15 (Suppl. 2), S74–S80. [Google Scholar] [CrossRef]
- Lorrot, M.; Doit, C.; Ilharreborde, B.; Vitoux, C.; Le Henaff, L.; Sebag, G.; Pennecot, G.; Grimprel, E.; Bingen, É. Antibiotic therapy of bone and joint infections in children: Recent changes. Arch. Pediatr. 2011, 18, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Grimprel, E. Pharmacokinetics and pharmacodynamics of antimicrobial therapy used in child osteoarticular infections. Arch. Pediatr. 2007, 14 (Suppl. 2), S122–S127. [Google Scholar] [CrossRef]
- Gerber, J.S.; Ross, R.K.; Bryan, M.; Localio, A.R.; Szymczak, J.E.; Wasserman, R.; Barkman, D.; Odeniyi, F.; Conaboy, K.; Bell, L.; et al. Association of Broad- vs Narrow-Spectrum Antibiotics with Treatment Failure, Adverse Events, and Quality of Life in Children with Acute Respiratory Tract Infections. JAMA 2017, 318, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Valour, F.; Trouillet-Assant, S.; Riffard, N.; Tasse, J.; Flammier, S.; Rasigade, J.P.; Chidiac, C.; Vandenesch, F.; Ferry, T.; Laurent, F. Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2029–2036. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of the Included Centers and Used Antibiotic Therapy | Total |
|---|---|
| Geographic location of the Center | |
| Northern Italy | 18 |
| Central Italy | 6 |
| Southern Italy | 6 |
| Island Regions | 1 |
| Number of children with acute hematogenous osteomyelitis (AHOM) followed per year by the Center | |
| <10 children | 21 |
| ≥10 children | 10 |
| First choice empirical intravenous antibiotics in children under 3 months of age | |
| Penicillin (Oxacillin, Ampicillin-Sulbactam, Amoxicillin-Clavulanate) | 3/31 (9.7%) |
| 3rd gen Cephalosporin | 1/31 (3.2%) |
| 3rd gen Cephalosporin + Aminoglycoside (Gentamicin, Netilmicin) | 3/31 (9.7%) |
| 3rd gen Cephalosporin + Rifampicin | 1/31 (3.2%) |
| 3rd gen Cephalosporin + Oxacillin | 7/31 (22.6%) |
| 3rd gen Cephalosporin + Glycopeptide | 6/31 (19.4%) |
| Penicillin (Ampicillin, Oxacillin) + Aminoglycoside (Gentamicin, Netilmicin) | 10/31 (32.3 %) |
| First choice empirical oral antibiotics in children under 3 months | |
| Never shift to oral therapy | 2/31 (6.5%) |
| Amoxicillin-Clavulanate | 20/31 (64.5%) |
| 3rd gen Cephalosporin | 3/31 (9.7%) |
| Clindamycin | 3/31 (9.7%) 3 |
| 2nd gen Cephalosporin + Rifampicin | 1/31 (3.2%) |
| Amoxicillin-Clavulanate + Rifampicin | 1/31 (3.2%) |
| Amoxicillin-Clavulanate + Clindamycin | 1/31 (3.2%) |
| First choice empirical intravenous antibiotics in children ≥3 months of age | |
| Penicillin (Oxacillin) | 6/31 (19.4%) |
| 1st gen Cephalosporin | 3/31 (9.7%) |
| 3rd gen Cephalosporin | 5/31 (16.1%) |
| Clindamycin | 1/31 (3.2%) |
| 3rd gen Cephalosporin + Glycopeptide | 5/31 (16.1%) |
| 3rd gen Cephalosporin + Oxacillin | 7/31 (22.6%) |
| 3rd gen Cephalosporin + Clindamycin | 3/31 (9.7%) |
| 3rd gen Cephalosporin + Rifampicin | 1/31 (3.2%) |
| First choice empirical oral antibiotics in children ≥3 months of age | |
| Amoxicillin-Clavulanate | 21/31 (67.7%) |
| 1st gen Cephalosporin | 1/31 (3.2%) |
| 3rd gen Cephalosporin | 1/31 (3.2%) |
| Clindamycin | 2/31 (6.5%) |
| Clarithromycin | 1/31 (3.2%) |
| Amoxicillin-Clavulanate + Rifampicin | 4/31 (12.9%) |
| Cotrimoxazole + Rifampicin | 1/31 (3.2%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, E.; Serrano, E.; Galli, L.; Villani, A.; Krzysztofiak, A.; Italian Paediatric Collaborative Osteomyelitis Study Group. Practical Issues in Early Switching from Intravenous to Oral Antibiotic Therapy in Children with Uncomplicated Acute Hematogenous Osteomyelitis: Results from an Italian Survey. Int. J. Environ. Res. Public Health 2019, 16, 3557. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16193557
Chiappini E, Serrano E, Galli L, Villani A, Krzysztofiak A, Italian Paediatric Collaborative Osteomyelitis Study Group. Practical Issues in Early Switching from Intravenous to Oral Antibiotic Therapy in Children with Uncomplicated Acute Hematogenous Osteomyelitis: Results from an Italian Survey. International Journal of Environmental Research and Public Health. 2019; 16(19):3557. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16193557
Chicago/Turabian StyleChiappini, Elena, Elena Serrano, Luisa Galli, Alberto Villani, Andrzej Krzysztofiak, and Italian Paediatric Collaborative Osteomyelitis Study Group. 2019. "Practical Issues in Early Switching from Intravenous to Oral Antibiotic Therapy in Children with Uncomplicated Acute Hematogenous Osteomyelitis: Results from an Italian Survey" International Journal of Environmental Research and Public Health 16, no. 19: 3557. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16193557





