Distribution, Ecological Risk Assessment, and Bioavailability of Cadmium in Soil from Nansha, Pearl River Delta, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Analysis
2.4. Soil Pollution Evaluation
2.4.1. Geo-Accumulation Index
2.4.2. Potential Ecological Risk Index (Er)
2.5. Multivariate Statistics and Spatial Analysis Methods
3. Results and Discussion
3.1. Spatial Distribution of Cd in Soil
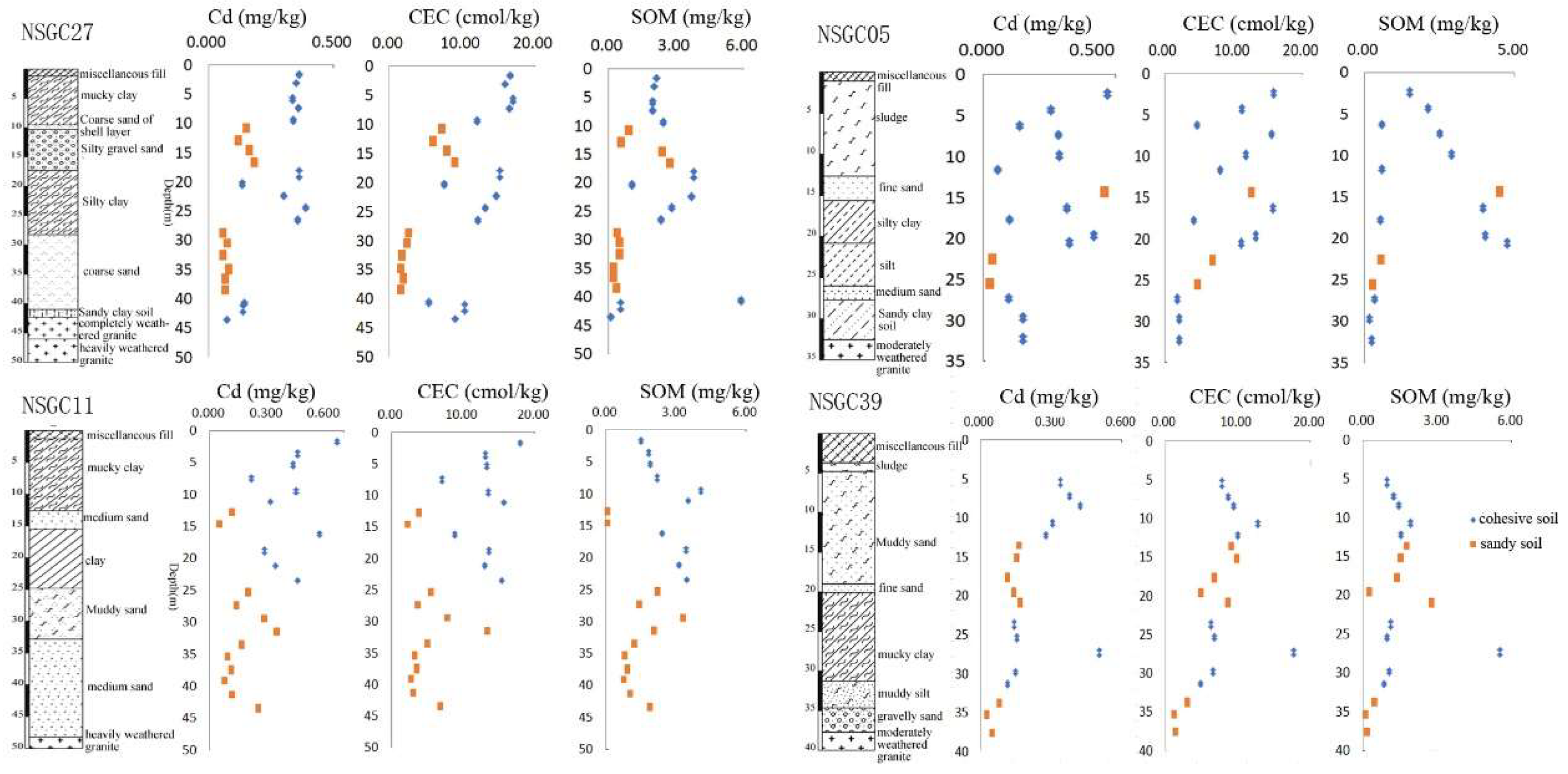
3.1.1. Vertical Distribution of Cd
Vertical Distribution of Cd in Deep Soil (0–200 cm)
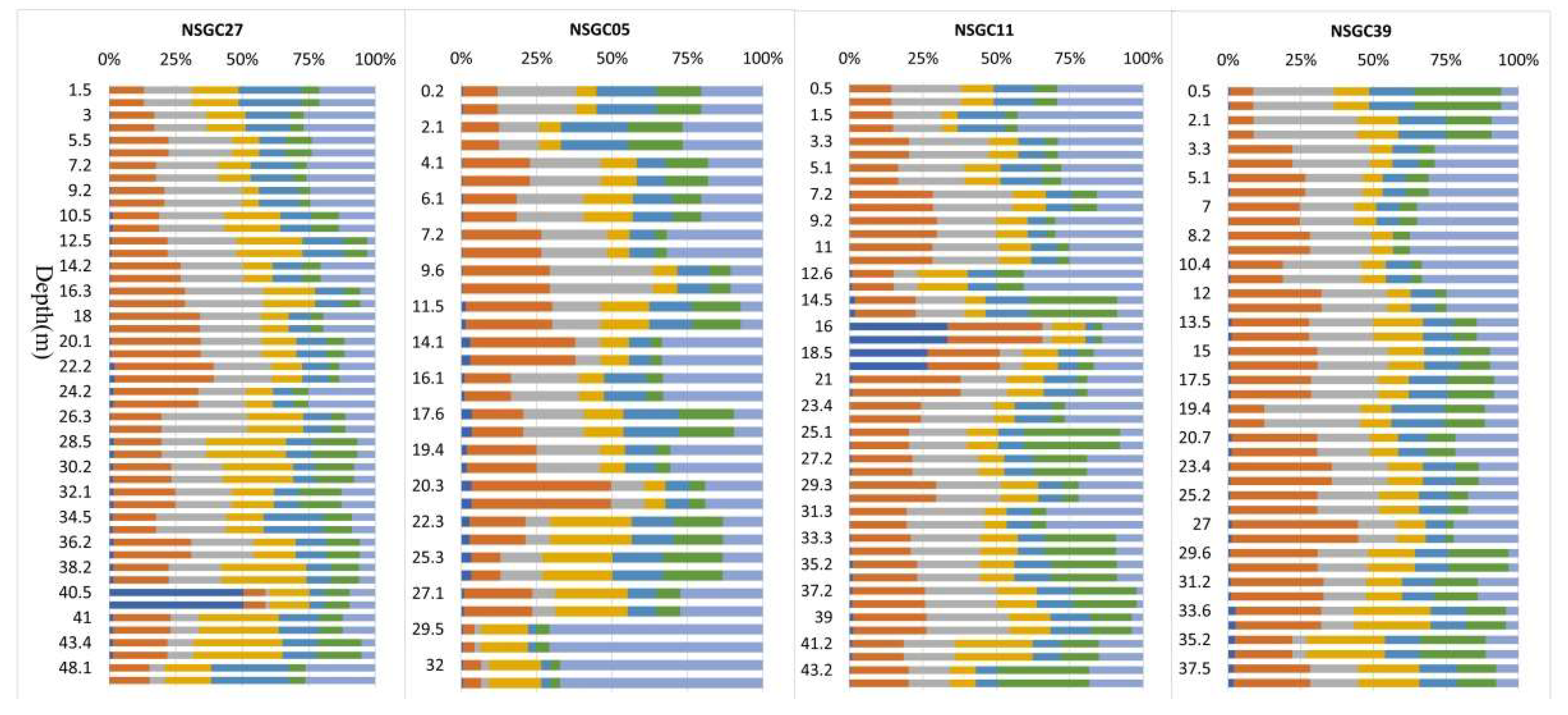
Vertical Distribution of Cd in Quaternary Sediments
3.1.2. The Plane Distribution Pattern for Cd in Topsoil (0–20 cm deep)
3.2. Factors that Influence Soil Cd Enrichment
3.2.1. Topography and the Quaternary Geological Conditions
3.2.2. Stream Transport
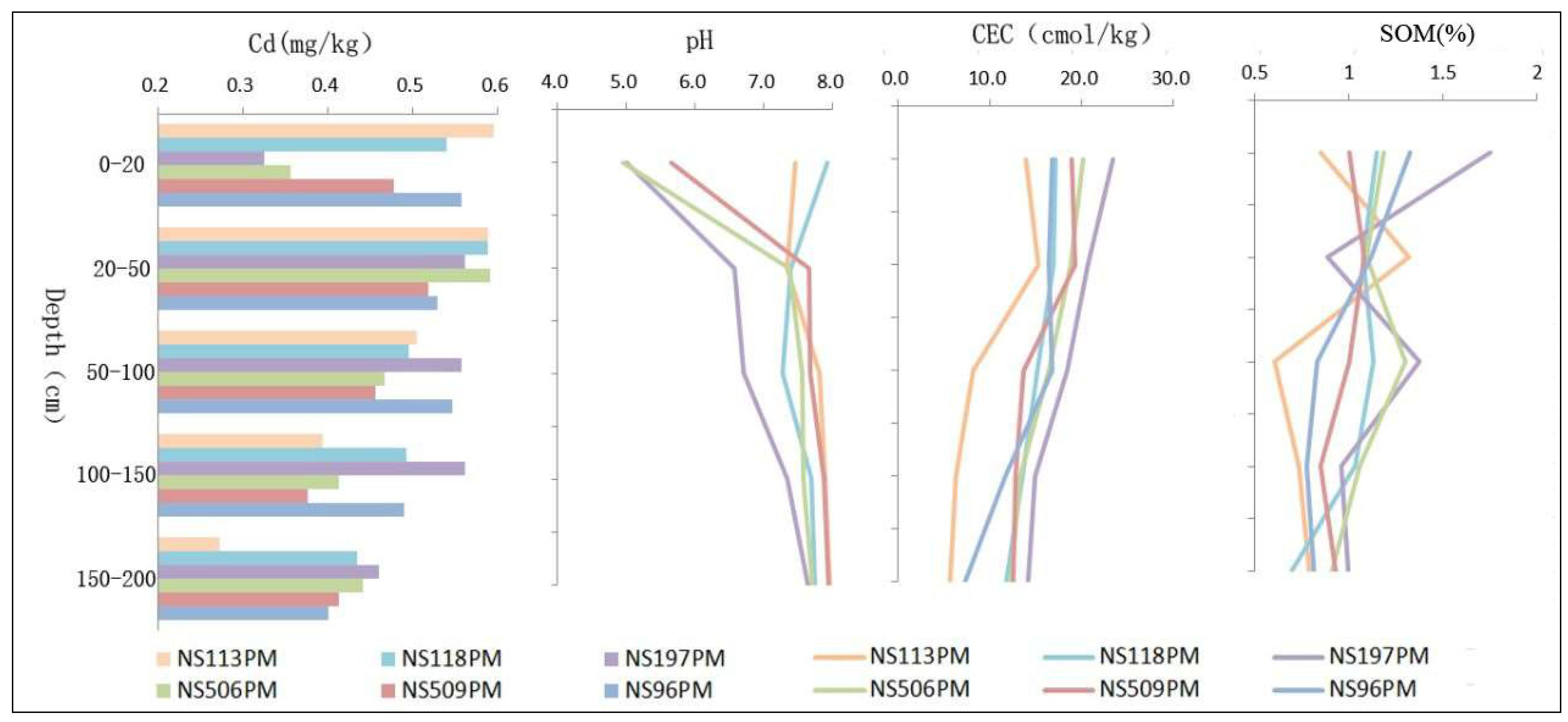
3.2.3. Physical and Chemical Properties of Soils
3.2.4. Human Engineering Activities
3.3. Evaluation of Soil Cd Pollution
3.4. Chemical Fractionation of Soil Cd
3.4.1. Cd Fractionation and Lability
3.4.2. Bioavailability of Cd across the Area
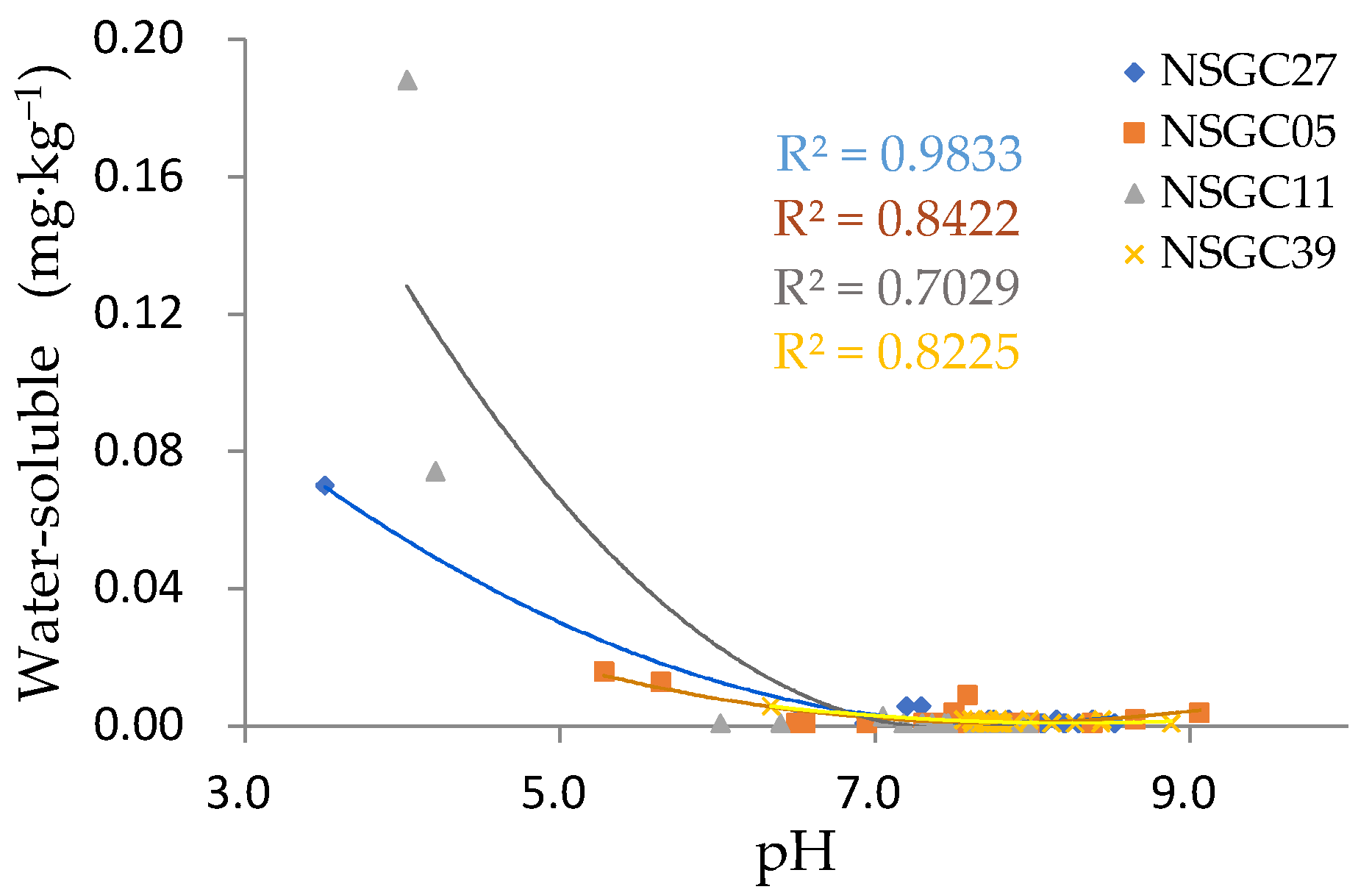
3.4.3. Factors Affecting Bioavailable Cd
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, F.; Zhang, J.D.; Huang, J.H.; Huang, D.; Yang, J.; Song, Y.; Zeng, G. Heavy metals in road dust from Xiandao District, Changsha City, China: Characteristics, health risk assessment, and integrated source identification. Environ. Sci. Pollut. Res. 2016, 23, 13100–13113. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Liu, R.; Chen, W.P.; Peng, C.; Markert, B. Effects of urbanization on heavy metal accumulation in surface soils, Beijing. J. Environ. Sci. 2018, 64, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, J.H.; Zeng, G.M.; Liu, W.; Huang, X.; Huang, B.; Gu, Y.; Shi, L.; He, X.; He, Y. Toxic metals in topsoil under different land uses from Xiandao District, middle China: Distribution, relationship with soil characteristics, and health risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 12261–12275. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, M.; Chen, W.P. Modelling cadmium contamination in paddy soils under long-term remediation measures: Model development and stochastic simulations. Environ. Pollut. 2016, 216, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.F.; Chen, T.B.; Lei, M.; Yang, J.; Guo, Q.J.; Song, B.; Zhou, X.Y. Spatial distribution of soil heavy metal pollution estimated by different interpolation methods: Accuracy and uncertainty analysis. Chemosphere 2011, 82, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Wang, C.Q.; Dai, T.F.; Shi, W.; Zhang, X.; Xiao, Y.; Song, W.; Li, B.; Wang, Y. Prediction of soil cadmium distribution across a typical area of Chengdu Plain, China. Sci. Rep. 2017, 7, 7115. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, Environmental Exposure, and Health Outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Dong, R.Z.; Jia, Z.M.; Li, S.Y. Risk assessment and sources identification of soil heavy metals in a typical county of Chongqing Municipality, Southwest China. Process Saf. Environ. Prot. 2018, 113, 275–281. [Google Scholar] [CrossRef]
- Huang, Y.; Li, T.Q.; Wu, C.X.; He, Z.; Japenga, J.; Deng, M.; Yang, X. An integrated approach to assess heavy metal source apportionment in pen-urban agricultural soils. J. Hazard. Mater. 2015, 299, 540–549. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, K.; Xu, J.-B.; Chen, G. Cadmium Pollution Situation, Harm, Source and Production Measures in Paddy Field of China. J. Anhui Agric. Sci. 2014, 42, 10540–10542. [Google Scholar]
- Zhang, H.; Luo, Y.; Zhang, H.; Song, J.; Xia, J.; Zhao, Q. Study on Soil Environmental Quality Guidelines and Standards V. Modeling of Cadmium Uptake in Soil-Crop Systems for Human Food Safety in China. Acta Pedol. Sin. 2010, 47, 628–638. [Google Scholar]
- Li, B.; Xiao, R.; Wang, C.Q.; Cao, L.; Zhang, Y.; Zheng, S.; Yang, L.; Guo, Y. Spatial distribution of soil cadmium and its influencing factors in peri-urban farmland: A case study in the Jingyang District, Sichuan, China. Environ. Monit. Assess. 2017, 189, 21. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.Q.; Bock, L.; Schvartz, C.; Colinet, G.; Li, Y. Mobility and distribution of lead, cadmium, copper and zinc in soil profiles in the peri-urban market garden of Kunming, Yunnan Province, China. Arch. Agron. Soil Sci. 2014, 60, 133–149. [Google Scholar] [CrossRef]
- Wang, D.F.; Wei, Z.Y.; Tang, S.M.; Qi, Z. Distribution of selenium and cadmium in soil-rice system of selenium-rich area in Hainan, China. Pak. J. Pharm. Sci. 2014, 27, 1633–1639. [Google Scholar] [PubMed]
- Agca, N.; Ozdel, E. Assessment of spatial distribution and possible sources of heavy metals in the soils of Sariseki-Dortyol District in Hatay Province (Turkey). Environ. Earth Sci. 2014, 71, 1033–1047. [Google Scholar] [CrossRef]
- Romic, M.; Romic, D. Heavy metals distribution in agricultural topsoils in urban area. Environ. Geol. 2003, 43, 795–805. [Google Scholar] [CrossRef]
- He, S.R.; Lu, Q.; Li, W.Y.; Ren, Z.; Zhou, Z.; Feng, X.; Zhang, Y.; Li, Y. Factors controlling cadmium and lead activities in different parent material-derived soils from the Pearl River Basin. Chemosphere 2017, 182, 509–516. [Google Scholar] [CrossRef]
- Liu, G.N.; Wang, J.; Xue, W.; Zhao, J.; Wang, J.; Liu, X. Effect of the size of variable charge soil particles on cadmium accumulation and adsorption. J. Soils Sediments 2017, 17, 2810–2821. [Google Scholar] [CrossRef]
- Kim, S.U.; Owens, V.N.; Kim, Y.G.; Lee, S.M.; Park, H.C.; Kim, K.K.; Son, H.J.; Hong, C.O. Effect of Phosphate Addition on Cadmium Precipitation and Adsorption in Contaminated Arable Soil with a Low Concentration of Cadmium. Bull. Environ. Contam. Toxicol. 2015, 95, 675–679. [Google Scholar] [CrossRef]
- Zhao, X.L.; Jiang, T.; Du, B. Effect of organic matter and calcium carbonate on behaviors of cadmium adsorption-desorption on/from purple paddy soils. Chemosphere 2014, 99, 41–48. [Google Scholar] [CrossRef]
- Jiang, H.; Li, T.Q.; Han, X.; Yang, X.; He, Z. Effects of pH and low molecular weight organic acids on competitive adsorption and desorption of cadmium and lead in paddy soils. Environ. Monit. Assess. 2012, 184, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Medina, I. Determination of diffusion coefficients for supercritical fluids. J. Chromatogr. A 2012, 1250, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Gallon, C.; Ranville, M.A.; Conaway, C.H.; Landing, W.M.; Buck, C.S.; Morton, P.L.; Flegal, A.R. Asian Industrial Lead Inputs to the North Pacific Evidenced by Lead Concentrations and Isotopic Compositions in Surface Waters and Aerosols. Environ. Sci. Technol. 2011, 45, 9874–9882. [Google Scholar] [CrossRef] [PubMed]
- Okin, G.S.; Parsons, A.J.; Wainwright, J.; Herrick, J.E.; Bestelmeyer, B.T.; Peters, D.C.; Fredrickson, E.L. Do Changes in Connectivity Explain Desertification? Bioscience 2009, 59, 237–244. [Google Scholar] [CrossRef]
- Filippelli, G.M.; Morrison, D.; Cicchella, D. Urban Geochemistry and Human Health. Elements 2012, 8, 439–444. [Google Scholar] [CrossRef]
- Csavina, J.; Landazuri, A.; Wonaschutz, A.; Rine, K.; Rheinheimer, P.; Barbaris, B.; Conant, W.; Sáez, A.E.; Betterton, E.A. Metal and Metalloid Contaminants in Atmospheric Aerosols from Mining Operations. Water Air Soil Pollut. 2011, 221, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Z. Recent progress in the study of heavy metal bioavailability in soil. Geophys. Geochem. Explor. 2014, 38, 1097–1106. [Google Scholar]
- Müller, G. Index of geoaccumulation in sediments of the Rhine rive. Geojournal 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Saeedi, M.; Jamshidi-Zanjani, A. Development of a new aggregative index to assess potential effect of metals pollution in aquatic sediments. Ecol. Indic. 2015, 58, 235–243. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Xiao, T.F.; Baveye, P.C.; Zhu, J.; Ning, Z.; Li, H. Potential health risk in areas with high naturally-occurring cadmium background in southwestern China. Ecotoxicol. Environ. Saf. 2015, 112, 122–131. [Google Scholar] [CrossRef]
- Cheng, S.W.; Liu, G.J.; Zhou, C.C.; Sun, R. Chemical speciation and risk assessment of cadmium in soils around a typical coal mining area of China. Ecotoxicol. Environ. Saf. 2018, 160, 67–74. [Google Scholar] [CrossRef]
- Yongming, H.; Peixuan, D.; Junji, C.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 2006, 355, 176–186. [Google Scholar] [CrossRef]
- Tahri, M.; Benyaich, F.; Bounakhla, M.; Bilal, E.; Gruffat, J.J.; Moutte, J.; Garcia, D. Multivariate analysis of heavy metal contents in soils, sediments and water in the region of Meknes (central Morocco). Environ. Monit. Assess. 2005, 102, 405–417. [Google Scholar] [CrossRef]
- Zahra, A.; Hashmi, M.Z.; Malik, R.N.; Ahmed, Z. Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah-Feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 2014, 470, 925–933. [Google Scholar] [CrossRef]
- Varol, M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 2011, 195, 355–364. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Cadmium Sorption and Desorption in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 489–533. [Google Scholar] [CrossRef]
- Lai, Q.; Yu, H.; Li, G.; Xia, B. Chemical Characteristics of Soil Cd-High Areas in Zhujiang Delta. Geophys. Geochem. Explor. 2005, 29, 334–335, 341. (In Chinese) [Google Scholar]
- Liao, J.; Chen, J.; Ru, X.; Chen, J.; Wu, H.; Wei, C. Heavy metals in river surface sediments affected with multiple pollution sources, South China: Distribution, enrichment and source apportionment. J. Geochem. Explor. 2017, 176, 9–19. [Google Scholar] [CrossRef]
- Dou, M.; Zuo, Q.T.; Zhang, J.P.; Li, C.; Li, G. Influence of changes in hydrodynamic conditions on cadmium transport in tidal river network of the Pearl River Delta, China. Environ. Monit. Assess. 2013, 185, 7501–7516. [Google Scholar] [CrossRef]
- Song, M.W.; Huang, P.; Li, F.; Zhang, H.; Xie, K.Z.; Wang, X.H.; He, G.X. Water quality of a tributary of the Pearl River, the Beijiang, Southern China: Implications from multivariate statistical analyses. Environ. Monit. Assess. 2011, 172, 589–603. [Google Scholar] [CrossRef]
- Tang, Z.; Hou, Q.; You, Y.; Yang, Z.; Li, K. Distribution Characteristics and Influencing Factors of Heavy Metals in Pearl River Delta Quaternary Boreholes. Adv. Earth Sci. 2017, 32, 885–898. [Google Scholar]
- Luo, X.; Guo, Q.; Xie, Z.; Yang, J.; Chai, Z.; Liu, X.; Wu, S. Study on heavy metal pollution in typical rural soils in Pearl River Delta area. Ecol. Environ. Sci. 2014, 23, 485–489. [Google Scholar]
- Li, F.; Zhang, J.; Liu, W.; Liu, J.; Huang, J.; Zeng, G. An exploration of an integrated stochastic-fuzzy pollution assessment for heavy metals in urban topsoil based on metal enrichment and bioaccessibility. Sci. Total Environ. 2018, 644, 649–660. [Google Scholar] [CrossRef]
- Xian, X. Effect of Chemical Forms of Cadmium, Zinc, and Lead in Polluted Soils on Their Uptake by Cabbage Plants. Plant Soil 1989, 113, 257–264. [Google Scholar] [CrossRef]
- Ma, L.Q.; Rao, G.N. Chemical fractionation of cadmium, copper, nickel, and zinc in contaminated soils. J. Environ. Qual. 1997, 26, 259–264. [Google Scholar] [CrossRef]
- Mao, M.; Liu, Z.; Dong, H.; Wang, H.; Shi, S.; Lin, Z.; Peng, X. Distribution and speciation of metals in sediments along Le An River. J. Environ. Sci. 1992, 4, 72–81. [Google Scholar]
- Ren, Z.L.; Tella, M.; Bravin, M.N.; Comans, R.N.J.; Dai, J.; Garnier, J.; Sivry, Y.; Doelsch, E.; Straathof, A.; Benedettia, M.F. Effect of dissolved organic matter composition on metal speciation in soil solutions. Chem. Geol. 2015, 398, 61–69. [Google Scholar] [CrossRef]







| Step | Fractions | Extract Composition | pH | Notes |
|---|---|---|---|---|
| F1 | Water soluble | 25 mL ultrapure water | pH = 8.0 ± 0.2 | 30 min ultrasound (40 KHz, 25 ± 5 °C), rinse with ultrapure H2O. |
| F2 | Exchangeable | 25 mL 1.0 mol/L MgCl2·6H2O | pH = 7.0 ± 0.2 | 30 min ultrasound (40 KHz, 25 ± 5 °C), rinse with ultrapure H2O. |
| F3 | Carbonate-bound | 25 mL 1.0 mol/L CH3COONa·3H2O | pH = 5.0 ± 0.2 | 60 min ultrasound (40 KHz, 25 ± 5 °C), rinse with ultrapure H2O. |
| F4 | Humic-acid-bound | 50 mL 0.1 mol/L Na4PO7·10H2O | pH = 10.0 ± 0.2 | 40 min ultrasound (40 KHz, 25 ± 5 °C), rinse with ultrapure H2O. |
| F5 | Fe–Mn-oxide-bound | 50 mL 0.25 mol/L HONH3Cl–HCl | 1 h ultrasound (40 KHz, 25 ± 5 °C), rinse with ultrapure H2O. | |
| F6 | Refractory-organic-matter-bound | 3 mL HNO3 + 5 mL 30% H2O2 | pH = 2.0 ± 0.2 | 1.5 h bath (83 °C, stirred every 10 min), another 1.0 h bath with 3 mL 30% H2O2 (83 °C, stirred every 10 min), rinse with ultrapure H2O. |
| F7 | Residual | 5 mL mixture of 37% HCl, 70% HClO4, and 70% HNO3 (1:1:1)/5 mL 40% HF | Digested at 105 °C for 3 h. |
| Boring Number | Number of Samples | MIN 1 | MAX 2 | AVG 3 | MID 4 | SD 5 | CV 6 |
|---|---|---|---|---|---|---|---|
| Unit: μg/Kg | |||||||
| NSGC27 | 24 | 55 | 388 | 209 | 160 | 126 | 0.601 |
| NSGC05 | 7 | 28 | 560 | 273 | 305 | 170 | 0.624 |
| NSGC11 | 23 | 54 | 664 | 299 | 286 | 167 | 0.577 |
| NSGC39 | 21 | 29 | 573 | 238 | 165 | 148 | 0.625 |
| Coastal sand (n = 4) | Fluvo-aquic soil (n = 47) | Red soil (n = 27) | |||||||
| pH | CEC | SOM | pH | CEC | SOM | pH | CEC | SOM | |
| MAX 1 | 8.30 | 5.52 | 4.05 | 8.28 | 19.65 | 12.17 | 8.43 | 13.81 | 6.87 |
| MIN 2 | 6.88 | 3.13 | 0.20 | 4.60 | 2.83 | 0.43 | 4.05 | 4.10 | 0.53 |
| AVG 3 | 7.59 | 4.51 | 1.82 | 6.76 | 14.29 | 2.46 | 5.89 | 7.06 | 2.62 |
| MID 4 | 7.59 | 4.69 | 1.51 | 6.92 | 16.22 | 2.19 | 5.44 | 6.63 | 2.45 |
| SD 5 | 0.64 | 0.87 | 1.60 | 1.04 | 4.71 | 1.62 | 1.19 | 2.35 | 1.38 |
| CV 6 | 0.08 | 0.19 | 0.88 | 0.15 | 0.33 | 0.66 | 0.20 | 0.33 | 0.53 |
| Paddy soil (n = 158) | Alluvial soil (n = 32) | Swamp soil (n = 77) | |||||||
| pH | CEC | SOM | pH | CEC | SOM | pH | CEC | SOM | |
| MAX 1 | 8.13 | 27.54 | 3.53 | 8.28 | 20.80 | 7.04 | 8.43 | 19.99 | 5.82 |
| MIN 2 | 4.28 | 7.98 | 0.77 | 4.63 | 4.50 | 0.00 | 5.84 | 5.94 | 0.55 |
| AVG 3 | 6.17 | 17.52 | 1.71 | 7.16 | 9.41 | 2.46 | 7.50 | 13.54 | 1.64 |
| MID 4 | 6.15 | 17.43 | 1.48 | 7.40 | 8.24 | 2.50 | 7.56 | 14.06 | 1.52 |
| SD 5 | 0.98 | 2.89 | 0.57 | 1.00 | 4.10 | 1.31 | 0.37 | 3.31 | 0.79 |
| CV 6 | 0.16 | 0.17 | 0.33 | 0.14 | 0.44 | 0.53 | 0.05 | 0.24 | 0.49 |
| Topsoil | NSGC27 | NSGC05 | NSGC11 | NSGC39 | ||
|---|---|---|---|---|---|---|
| Correlation coefficient | CEC | 0.159 | 0.912 | 0.760 | 0.805 | 0.820 |
| pH | 0.250 | −0.124 | −0.177 | −0.155 | −0.333 | |
| SOM | 0.171 | 0.503 | 0.741 | 0.499 | 0.552 | |
| Sig (single side) | CEC | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| pH | 0.000 | 0.200 | 0.159 | 0.152 | 0.016 | |
| SOM | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Boring Number | Bioavailable Cd | Moderately Bioavailable Cd | ||||||
|---|---|---|---|---|---|---|---|---|
| NSGC27 | NSGC05 | NSGC11 | NSGC39 | NSGC27 | NSGC05 | NSGC11 | NSGC39 | |
| MAX | 0.127 | 0.202 | 0.370 | 0.220 | 0.242 | 0.336 | 0.277 | 0.308 |
| MIN | 0.012 | 0.004 | 0.013 | 0.010 | 0.039 | 0.022 | 0.038 | 0.029 |
| AVG | 0.051 | 0.062 | 0.077 | 0.060 | 0.117 | 0.128 | 0.137 | 0.123 |
| 0–20 m AVG | 0.056 | 0.084 | 0.104 | 0.063 | 0.146 | 0.165 | 0.157 | 0.154 |
| >30 m AVG | 0.029 | 0.010 | 0.035 | 0.022 | 0.069 | 0.045 | 0.100 | 0.043 |
| Fractions | NSGC27 | NSGC05 | NSGC11 | NSGC39 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Bioavailable | Labile | Total | Bioavailable | Labile | Total | Bioavailable | Labile | Total | Bioavailable | Labile | |
| Total | 1.00 | 0.780 | 0.893 | 1.00 | 0.773 | 0.891 | 1.00 | 0.679 | 0.898 | 1.00 | 0.787 | 0.977 |
| Bioavailable | 0.780 | 1.00 | 0.916 | 0.773 | 1.00 | 0.920 | 0.679 | 1.00 | 0.908 | 0.787 | 1.00 | 0.885 |
| Labile | 0.893 | 0.916 | 1.00 | 0.891 | 0.920 | 1.00 | 0.898 | 0.908 | 1.00 | 0.977 | 0.885 | 1.00 |
| Water-soluble | −0.064 | 0.266 | 0.030 | 0.555 | 0.876 | 0.696 | 0.345 | 0.877 | 0.635 | 0.390 | 0.674 | 0.478 |
| Exchangeable | 0.827 | 0.917 | 0.928 | 0.782 | 0.999 | 0.928 | 0.841 | 0.892 | 0.962 | 0.790 | 1.00 | 0.887 |
| Carbonate | 0.846 | 0.653 | 0.902 | 0.774 | 0.478 | 0.783 | 0.749 | 0.121 | 0.526 | 0.895 | 0.457 | 0.819 |
| Humic acid | 0.756 | 0.397 | 0.534 | 0.843 | 0.640 | 0.671 | 0.831 | 0.747 | 0.869 | 0.843 | 0.592 | 0.809 |
| Fe–Mn oxide | 0.773 | 0.314 | 0.430 | 0.778 | 0.331 | 0.541 | 0.795 | 0.170 | 0.484 | 0.674 | 0.243 | 0.582 |
| Organic | 0.796 | 0.527 | 0.620 | 0.622 | 0.174 | 0.364 | −0.004 | −0.202 | −0.164 | 0.388 | −0.084 | 0.233 |
| Residual | 0.932 | 0.655 | 0.746 | 0.800 | 0.539 | 0.558 | 0.893 | 0.339 | 0.633 | 0.868 | 0.777 | 0.869 |
| Fractionation | % Total (%) | Variation Coefficients (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| NSGC27 | NSGC05 | NSGC11 | NSGC39 | NSGC27 | NSGC05 | NSGC11 | NSGC39 | |
| Water-soluble | 3.07 | 1.44 | 3.15 | 0.89 | 321.79 | 83.19 | 264.81 | 70.9 |
| Exchangeable | 22.13 | 20.31 | 22.69 | 25.65 | 31.5 | 51.47 | 25.69 | 32.81 |
| Carbonate | 20.43 | 16.17 | 19.86 | 20.95 | 35.29 | 53.01 | 32.56 | 33.04 |
| Humic acid | 18.06 | 13.37 | 11.56 | 12.93 | 42.07 | 47.76 | 35.56 | 44.02 |
| Fe–Mn oxide | 13.01 | 11.86 | 10.22 | 10.82 | 42.07 | 45.07 | 29.66 | 33.37 |
| Organic | 8.83 | 10.2 | 12.3 | 11.39 | 43.99 | 57.61 | 79.45 | 59.94 |
| Residual | 14.46 | 26.66 | 20.22 | 17.36 | 53.79 | 65.73 | 53.56 | 59.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Huang, C.; Chen, Z.; Bao, K. Distribution, Ecological Risk Assessment, and Bioavailability of Cadmium in Soil from Nansha, Pearl River Delta, China. Int. J. Environ. Res. Public Health 2019, 16, 3637. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16193637
Wang F, Huang C, Chen Z, Bao K. Distribution, Ecological Risk Assessment, and Bioavailability of Cadmium in Soil from Nansha, Pearl River Delta, China. International Journal of Environmental Research and Public Health. 2019; 16(19):3637. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16193637
Chicago/Turabian StyleWang, Fangting, Changsheng Huang, Zhihua Chen, and Ke Bao. 2019. "Distribution, Ecological Risk Assessment, and Bioavailability of Cadmium in Soil from Nansha, Pearl River Delta, China" International Journal of Environmental Research and Public Health 16, no. 19: 3637. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16193637




