Change in the Distribution of Phosphorus Fractions in Aggregates under Different Land Uses: A Case in Sanjiang Plain, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Location
2.2. Soil Aggregate Segregation
2.3. Phosphorus Sorption Isotherm Experiment
2.4. Phosphorus Sequential Fractionation
2.5. Chemical Determination
2.6. Statistical Analysis
3. Results and Analysis
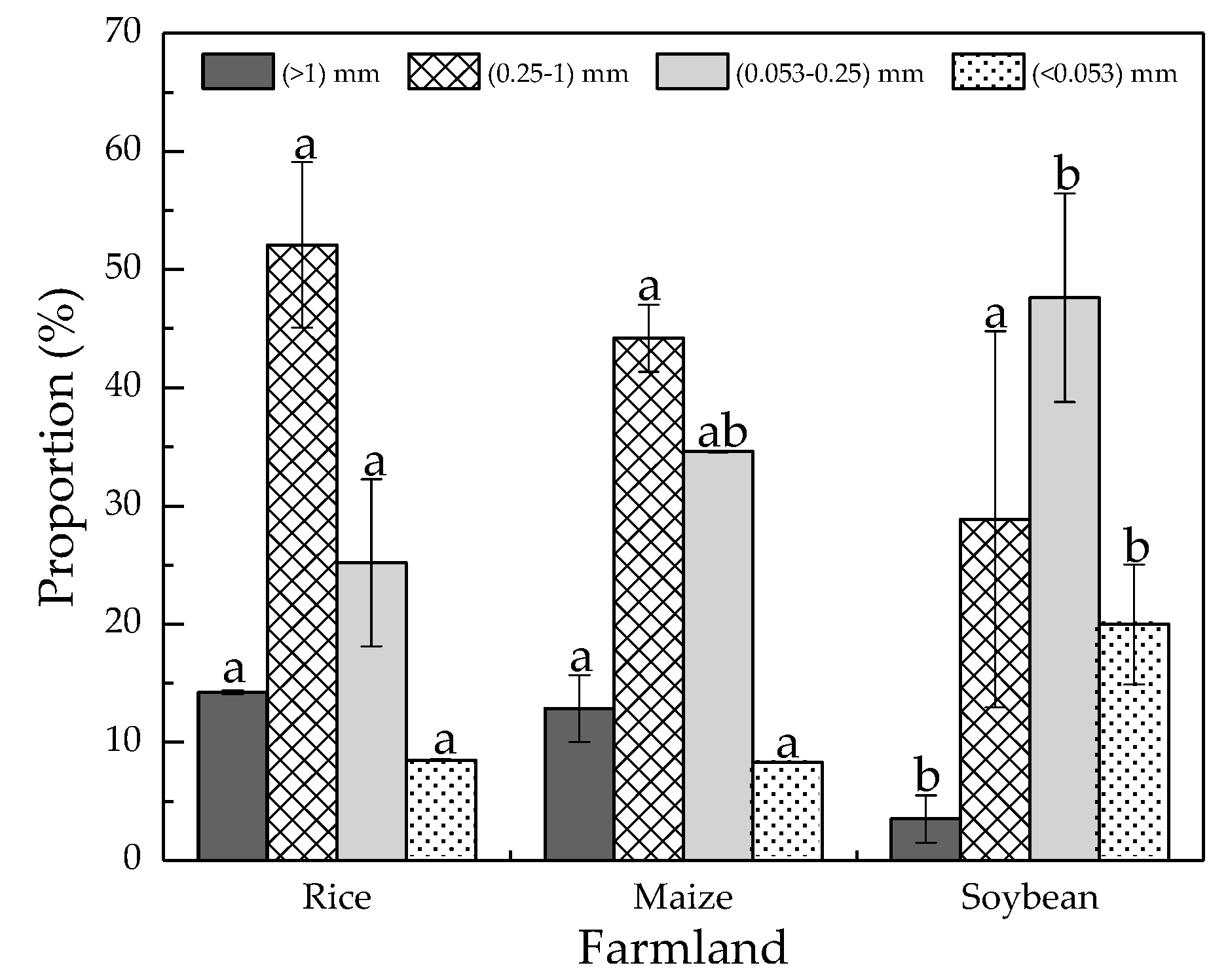
3.1. Soil Aggregates
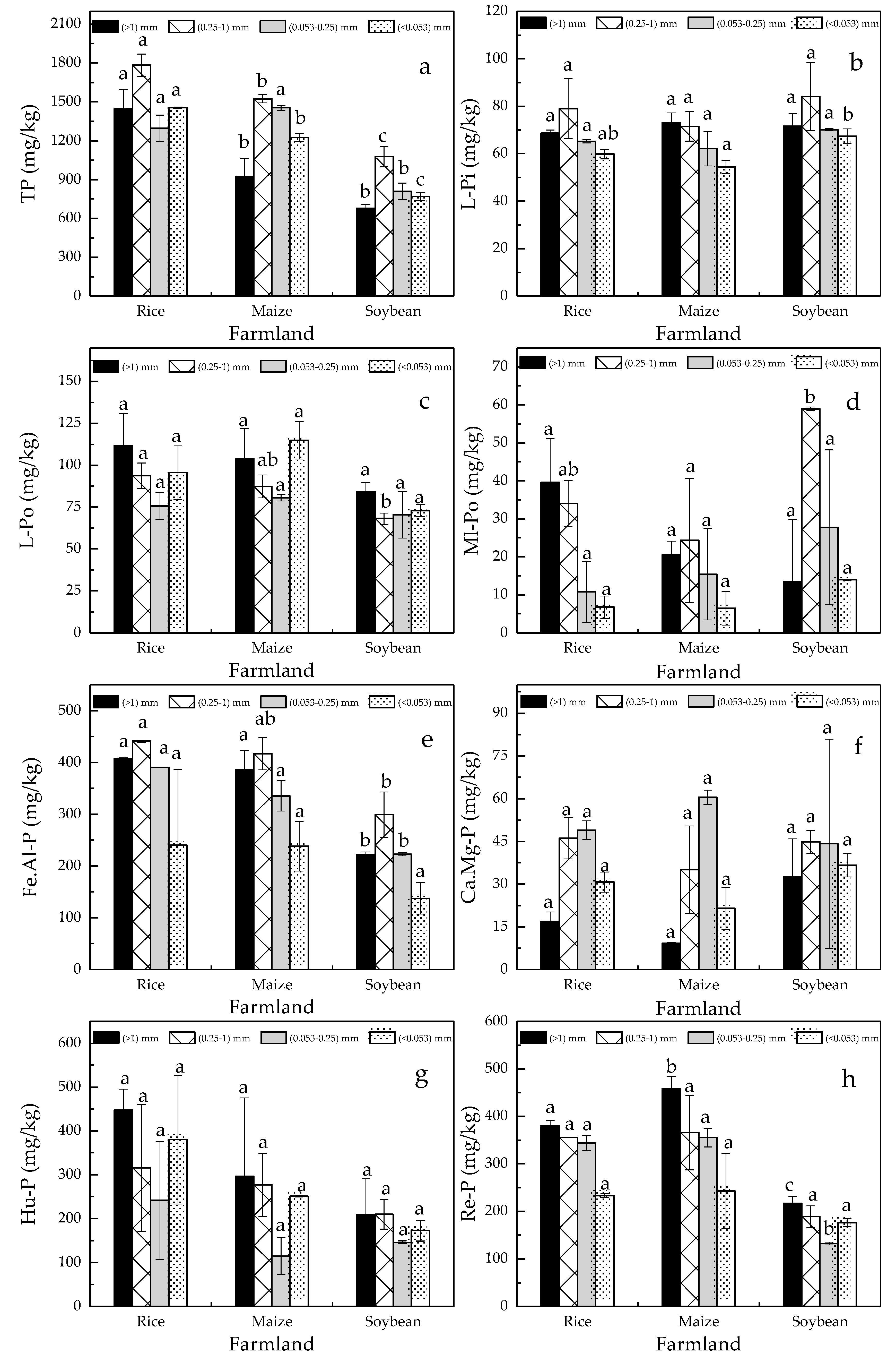
3.2. Phosphorus Fractions
3.3. Phosphorus Adsorption Characteristic
4. Discussion
4.1. Identification of Environmental Controlling Factors of Phosphorus Fractions Distribution
4.2. Phosphorus Availability and Release under Different Cultivated Crops
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.H.; Wen, X.W.; Wang, B.; Nie, P.Y. Agricultural pollution and regulation: How to subsidize agriculture? J. Clean. Prod. 2017, 164, 258–264. [Google Scholar] [CrossRef]
- Ouyang, W.; Song, K.; Wang, X.; Hao, F. Non-point source pollution dynamics under long-term agricultural development and relationship with landscape dynamics. Ecol. Indic. 2014, 45, 579–589. [Google Scholar] [CrossRef]
- Ni, X.; Parajuli, P.B. Evaluation of the impacts of BMPs and tailwater recovery system on surface and groundwater using satellite imagery and SWAT reservoir function. Agric. Water Manag. 2018, 210, 78–87. [Google Scholar] [CrossRef]
- Ouyang, W.; Xu, Y.; Cao, J.; Gao, X.; Gao, B.; Hao, Z.; Lin, C. Rainwater characteristics and interaction with atmospheric particle matter transportation analyzed by remote sensing around Beijing. Sci. Total Environ. 2019, 651 Pt 1, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, S.; Ma, W.; Roelcke, M.; Nieder, R.; Shi, S.; Wu, J.; Zhang, F. Changes in nitrogen and phosphorus flows and losses in agricultural systems of three megacities of China, 1990–2014. Resour. Conserv. Recycl. 2018, 139, 64–75. [Google Scholar] [CrossRef]
- Kaiserli, A.; Voutsa, D.; Samara, C. Phosphorus fractionation in lake sediments—Lakes Volvi and Koronia, N. Greece. Chemosphere 2002, 46, 1147–1155. [Google Scholar] [CrossRef]
- Jin, X.; He, Y.; Kirumba, G.; Hassan, Y.; Li, J. Phosphorus fractions and phosphate sorption-release characteristics of the sediment in the Yangtze River estuary reservoir. Ecol. Eng. 2013, 55, 62–66. [Google Scholar] [CrossRef]
- Fytianos, K.; Kotzakioti, A. Sequential fractionation of phosphorus in lake sediments of Northern Greece. Environ. Monit. Assess. 2005, 100, 191–200. [Google Scholar] [CrossRef]
- Cui, Y.; Xiao, R.; Xie, Y.; Zhang, M. Phosphorus fraction and phosphate sorption-release characteristics of the wetland sediments in the Yellow River Delta. Phys. Chem. Earth 2018, 103, 19–27. [Google Scholar] [CrossRef]
- Cavalcante, H.; Araujo, F.; Noyma, N.P.; Becker, V. Phosphorus fractionation in sediments of tropical semiarid reservoirs. Sci. Total Environ. 2018, 619, 1022–1029. [Google Scholar] [CrossRef]
- Kwak, D.-H.; Jeon, Y.-T.; Hur, Y.D. Phosphorus fractionation and release characteristics of sediment in the Saemangeum Reservoir for seasonal change. Int. J. Sediment Res. 2018, 33, 250–261. [Google Scholar] [CrossRef]
- Maharjan, M.; Maranguit, D.; Kuzyakov, Y. Phosphorus fractions in subtropical soils depending on land use. Eur. J. Soil Biol. 2018, 87, 17–24. [Google Scholar] [CrossRef]
- Ajmone-Marsan, F.; Cote, D.; Simard, R.R. Phosphorus transformations under reduction in long-term manured soils. Plant Soil 2006, 282, 239–250. [Google Scholar] [CrossRef]
- Adhikari, P.L.; White, J.R.; Maiti, K.; Nhan, N. Phosphorus speciation and sedimentary phosphorus release from the Gulf of Mexico sediments: Implication for hypoxia. Estuar. Coast. Shelf Sci. 2015, 164, 77–85. [Google Scholar] [CrossRef]
- Pan, F.; Liu, H.; Guo, Z.; Li, Z.; Wang, B.; Gao, A. Geochemical behavior of phosphorus and iron in porewater in a mangrove tidal flat and associated phosphorus input into the ocean. Cont. Shelf Res. 2017, 150, 65–75. [Google Scholar] [CrossRef]
- Surridge, B.W.J.; Heathwaite, A.L.; Baird, A.J. Phosphorus mobilisation and transport within a long-restored floodplain wetland. Ecol. Eng. 2012, 44, 348–359. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Kaul, H.-P.; Vollmann, J.; Eitzinger, J.; Wenzel, W. Developing phosphorus-efficient crop varieties-An interdisciplinary research framework. Field Crops Res. 2014, 162, 87–98. [Google Scholar] [CrossRef]
- Ranatunga, T.D.; Reddy, S.S.; Taylor, R.W. Phosphorus distribution in soil aggregate size fractions in a poultry litter applied soil and potential environmental impacts. Geoderma 2013, 192, 446–452. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.; Zhu, A.; Zhang, J.; Chen, L. Application of P-31 NMR spectroscopy in determining phosphatase activities and P composition in soil aggregates influenced by tillage and residue management practices. Soil Tillage Res. 2014, 138, 35–43. [Google Scholar] [CrossRef]
- Li, F.; Xue, C.; Qiu, P.; Li, Y.; Shi, J.; Shen, B.; Yang, X.; Shen, Q. Soil aggregate size mediates the responses of microbial communities to crop rotation. Eur. J. Soil Biol. 2018, 88, 48–56. [Google Scholar] [CrossRef]
- Remenant, B.; Grundmann, G.L.; Jocteur-Monrozier, L. From the micro-scale to the habitat: Assessment of soil bacterial community structure as shown by soil structure directed sampling. Soil Biol. Biochem. 2009, 41, 29–36. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. A time for every season: Soil aggregate turnover stimulates decomposition and reduces carbon loss in grasslands managed for bioenergy. Glob. Chang. Biol. Bioenergy 2016, 8, 588–599. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Gueldner, D.; Krausmann, F. Nutrient recycling and soil fertility management in the course of the industrial transition of traditional, organic agriculture: The case of Bruck estate, 1787–1906. Agric. Ecosyst. Environ. 2017, 249, 80–90. [Google Scholar] [CrossRef]
- Wang, R.; Dorodnikov, M.; Dijkstra, F.A.; Yang, S.; Xu, Z.; Li, H.; Jiang, Y. Sensitivities to nitrogen and water addition vary among microbial groups within soil aggregates in a semiarid grassland. Biol. Fertil. Soils 2017, 53, 129–140. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol. Oceanogr. 1992, 37, 1460–1482. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Zhong, X.; Lin, F.; Liu, C.; Yang, L.; Wang, M.; Chen, G.; Chen, Y.; Yang, Y. Responses of soil phosphorus fractions after nitrogen addition in a subtropical forest ecosystem: Insights from decreased Fe and Al oxides and increased plant roots. Geoderma 2019, 337, 246–255. [Google Scholar] [CrossRef]
- Weand, M.P.; Arthur, M.A.; Lovett, G.M.; Sikora, F.; Weathers, K.C. The phosphorus status of northern hardwoods differs by species but is unaffected by nitrogen fertilization. Biogeochemistry 2010, 97, 159–181. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Gu, J.; Yu, L.; Wang, Z. Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larixgmelinii plantation. Ann. For. Sci. 2015, 72, 435–442. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, G.; Liu, J.; Duan, H.; Liu, X.; Fang, X.; Zhang, D. Shifts in soil phosphorus fractions under elevated CO2 and N addition in model forest ecosystems in subtropical China. Plant Ecol. 2014, 215, 1373–1384. [Google Scholar] [CrossRef]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Lang, F.; Neal, A.L.; Stutter, M.I.; Almeida, D.S.; et al. Organic phosphorus in the terrestrial environment: A perspective on the state of the art and future priorities. Plant Soil 2018, 427, 191–208. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Maggio, R.M.; Cerretani, L. Chemometric applications to assess quality and critical parameters of virgin and extra-virgin olive oil. A review. Anal. Chim. Acta 2016, 913, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Six, J. Phosphorus cycling within soil aggregate fractions of a highly weathered tropical soil: A conceptual model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.H.; Zhang, X.P.; Liang, W.J.; Chen, L.J. Tillage effects on phosphorus composition and phosphatase activities in soil aggregates. Geoderma 2014, 217–218, 37–44. [Google Scholar] [CrossRef]
- Gu, S.; Gruau, G.; Dupas, R.; Rumpel, C.; Crème, A.; Fovet, O.; Gascuel-Odoux, C.; Jeanneau, L.; Humbert, G.; Petitjean, P. Release of dissolved phosphorus from riparian wetlands: Evidence for complex interactions among hydroclimate variability, topography and soil properties. Sci. Total Environ. 2017, 598, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.C.; Wang, S.R.; Pang, Y.; Wu, F.C. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environ. Pollut. 2006, 139, 288–295. [Google Scholar] [CrossRef]
- Kim, L.H.; Choi, E.; Stenstrom, M.K. Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 2003, 50, 53–61. [Google Scholar] [CrossRef]
- Reed, S.C.; Townsend, A.R.; Taylor, P.G.; Cleveland, C.C. Phosphorus Cycling in Tropical Forests Growing on Highly Weathered Soils. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 339–369. [Google Scholar]
- Maranguit, D.; Guillaume, T.; Kuzyakov, Y. Land-use change affects phosphorus fractions in highly weathered tropical soils. Catena 2017, 149, 385–393. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.; Sun, X.; Wen, W. Phosphorus fractions and its release in the sediments of Haihe River, China. J. Environ. Sci. 2009, 21, 291–295. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, Y.; Li, M.; Whelan, M.J. Microbial community composition and activity controls phosphorus transformation in rhizosphere soils of the Yeyahu Wetland in Beijing, China. Sci. Total Environ. 2018, 628–629, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Bastami, K.D.; Neyestani, M.R.; Raeisi, H.; Shafeian, E.; Baniamam, M.; Shirzadi, A.; Esmaeilzadeh, M.; Mozaffari, S.; Shahrokhi, B. Bioavailability and geochemical speciation of phosphorus in surface sediments of the Southern Caspian Sea. Mar. Pollut. Bull. 2018, 126, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bhadha, J.H.; Harris, W.G.; Jawitz, J.W. Soil Phosphorus Release and Storage Capacity from an Impacted Subtropical Wetland. Soil Sci. Soc. Am. J. 2010, 74, 1816–1825. [Google Scholar] [CrossRef]
- Ruban, V.; Lopez-Sanchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Fresenius J. Anal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Amirbahman, A.; Lake, B.A.; Norton, S.A. Seasonal phosphorus dynamics in the surficial sediment of two shallow temperate lakes: A solid-phase and pore-water study. Hydrobiologia 2013, 701, 65–77. [Google Scholar] [CrossRef]
- Ruiz-Fernandez, A.C.; Hillaire-Marcel, C.; Ghaleb, B.; Soto-Jimenez, M.; Paez-Osuna, F. Recent sedimentary history of anthropogenic impacts on the Culiacan River Estuary, northwestern Mexico: Geochemical evidence from organic matter and nutrients. Environ. Pollut. 2002, 118, 365–377. [Google Scholar] [CrossRef]


| Farmland | pH | EC | TN | TP | SOM | Fe | Mn |
|---|---|---|---|---|---|---|---|
| - | µs/cm | mg/kg | mg/kg | g/kg | g/kg | g/kg | |
| Rice | 5.29 ± 0.21 a | 82.45 ± 9.98 a | 1802.75 ± 176.42 a | 792.46 ± 175.97 a | 47.55 ± 9.83 a | 35.96 ± 1.02 a | 1.34 ± 0.40 a |
| Maize | 5.21 ± 0.04 a | 57.29 ± 6.25 a | 1715.50 ± 94.05 a | 1052.54 ± 341.70 a | 44.15 ± 1.34 a | 41.92 ± 3.85 a | 1.27 ± 0.13 a |
| Soybean | 5.34 ± 0.01 a | 78.89 ± 6.93 a | 1016.25 ± 238.6 b | 893.60 ± 24.91 a | 28.05 ± 5.30 a | 20.34 ± 1.44 b | 0.22 ± 0.02 b |
| Soil Aggregate Fractions | Farmland | Langmuir [Ce/Qe = Ce/Qm + 1/(K1·Qm)] | Freundlich [lgQe = lgK2 + 1/n·lgCe] | |||
|---|---|---|---|---|---|---|
| Qm | MBC | K1 | R2 | R2 | ||
| mm | - | mg/kg | mg/kg | - | - | - |
| >1 | Rice | 2020.41 ± 28.86 a | 2239.82 ± 991.87 a | 1.11 ± 0.51 a | 0.930 | 0.266 |
| Maize | 2037.04 ± 261.89 a | 1605.39 ± 502.60 a | 0.81 ± 0.35 a | 0.917 | 0.276 | |
| Soybean | 1923.08 ± 0.00 a | 654.01 ± 78.08 a | 0.34 ± 0.04 a | 0.876 | 0.008 | |
| 0.25–1 | Rice | 1562.50 ± 0.00 a | 1204.98 ± 406.66 a | 0.77 ± 0.26 a | 0.985 | 0.103 |
| Maize | 1440.71 ± 73.29 a | 872.95 ± 238.61 a | 0.61 ± 0.20 a | 0.982 | 0.002 | |
| Soybean | 1120.15 ± 79.65 b | 266.42 ± 28.45 a | 0.24 ± 0.04 a | 0.949 | 0.135 | |
| 0.053–0.25 | Rice | 1590.91 ± 107.14 a | 520.07 ± 101.32 a | 0.33 ± 0.09 a | 0.888 | 0.044 |
| Maize | 1399.22 ± 41.51 a | 357.13 ± 36.78 a | 0.26 ± 0.03 a | 0.806 | 0.299 | |
| Soybean | 594.23 ± 54.70 b | 229.31 ± 58.96 a | 0.39 ± 0.14 a | 0.960 | 0.057 | |
| <0.053 | Rice | 1459.93 ± 15.07 a | 395.60 ± 29.79 a | 0.25 ± 0.02 a | 0.903 | 0.224 |
| Maize | 583.13 ± 7.21 b | 487.37 ± 55.07 a | 0.21 ± 0.02 a | 0.998 | 0.201 | |
| Soybean | 676.45 ± 183.85 b | 149.06 ± 28.09 b | 0.68 ± 0.13 b | 0.900 | 0.277 | |
| Chemical Properties | L-Pi | L-Po | Ml-Po | Fe.Al-P | Ca.Mg-P | Hu-P | Re-P | TP |
|---|---|---|---|---|---|---|---|---|
| TN | 0.125 | 0.577 ** | 0.109 | 0.766 ** | −0.292 | 0.405 * | 0.786 ** | 0.497 * |
| SOM | −0.019 | 0.547 ** | 0.123 | 0.566 ** | −0.314 | 0.499 * | 0.655 ** | 0.339 |
| Fe | −0.072 | 0.451 * | −0.132 | 0.626 ** | −0.261 | 0.231 | 0.771 ** | 0.484 * |
| Mn | 0.212 | 0.457 * | 0.138 | 0.742 ** | −0.371 | 0.463 ** | 0.862 ** | 0.486 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Ou, Y.; Wang, L.; Yan, B.; Han, L.; Li, Y. Change in the Distribution of Phosphorus Fractions in Aggregates under Different Land Uses: A Case in Sanjiang Plain, Northeast China. Int. J. Environ. Res. Public Health 2019, 16, 212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16020212
Cui H, Ou Y, Wang L, Yan B, Han L, Li Y. Change in the Distribution of Phosphorus Fractions in Aggregates under Different Land Uses: A Case in Sanjiang Plain, Northeast China. International Journal of Environmental Research and Public Health. 2019; 16(2):212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16020212
Chicago/Turabian StyleCui, Hu, Yang Ou, Lixia Wang, Baixing Yan, Lu Han, and Yingxin Li. 2019. "Change in the Distribution of Phosphorus Fractions in Aggregates under Different Land Uses: A Case in Sanjiang Plain, Northeast China" International Journal of Environmental Research and Public Health 16, no. 2: 212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16020212