The Effect of Prenatal Stress, Proxied by Marital and Paternity Status, on the Risk of Preterm Birth
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
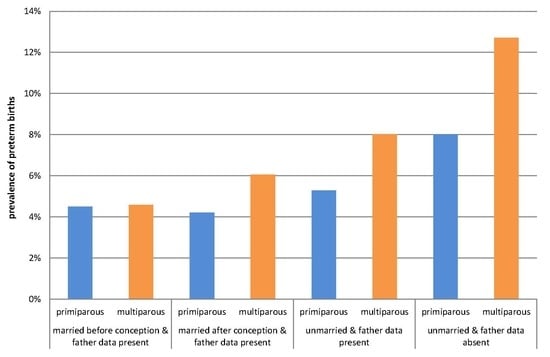
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parity Strata | RR | RR − 95% CI | RR + 95% CI | RR | RR − 95% CI | RR + 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted * | |||||||
| All | primiparous | [ref.cat.] | 1.00 | 1.00 | ||||
| multiparous | 1.09 | 1.03 | 1.16 | 0.90 | 0.84 | 0.97 | ||
| Crude | Adjusted ** | |||||||
| MBC-FS | primiparous | [ref.cat.] | 1.00 | 1.00 | ||||
| multiparous | 1.02 | 0.95 | 1.10 | 0.78 | 0.72 | 0.85 | ||
| MAC-FS | primiparous | [ref.cat.] | 1.00 | 1.00 | ||||
| multiparous | 1.44 | 1.15 | 1.81 | 1.28 | 0.99 | 1.65 | ||
| UM-FS | primiparous | [ref.cat.] | 1.00 | 1.00 | ||||
| multiparous | 1.52 | 1.29 | 1.79 | 1.21 | 1.01 | 1.46 | ||
| UM-NFS | primiparous | [ref.cat.] | 1.00 | 1.00 | ||||
| multiparous | 1.61 | 1.32 | 1.96 | 1.37 | 1.07 | 1.74 |
References
- Braveman, P.A.; Egerter, S.A.; Mockenhaupt, R.E. Broadening the focus: The need to address the social determinants of health. Am. J. Prev. Med. 2011, 40 (Suppl. 1), S4–S18. [Google Scholar] [CrossRef] [PubMed]
- Lantz, P.M.; House, J.S.; Mero, R.P.; Williams, D.R. Stress, life events, and socioeconomic disparities in health: Results from the Americans’ Changing Lives Study. J. Health Soc. Behav. 2005, 46, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, A.M.; Lundsberg, L.S.; Miller, D.; Stanwood, N.L.; Yonkers, K.A. Are pregnancy planning and pregnancy timing associated with maternal psychiatric illness, psychological distress and support during pregnancy? J. Affect. Disord. 2016, 205, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardwell, M.S. Stress: Pregnancy considerations. Obstet. Gynecol. Surv. 2013, 68, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Glynn, L.M.; Schetter, C.D.; Hobel, C.J.; Sandman, C.A. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008, 27, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, S.; Hennessy, M.D. Psychological and physiological stress: Impact on preterm birth. J. Obstet. Gynecol. Neonatal Nurs. 2003, 32, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Dunkel Schetter, C.; Tanner, L. Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Curr. Opin. Psychiatry 2012, 25, 141–148. [Google Scholar] [CrossRef]
- Lancaster, C.A.; Gold, K.J.; Flynn, H.A.; Yoo, H.; Marcus, S.M.; Davis, M.M. Risk factors for depressive symptoms during pregnancy: A systematic review. Am. J. Obstet. Gynecol. 2010, 202, 5–14. [Google Scholar] [CrossRef]
- Morse, C.A.; Buist, A.; Durkin, S. First-time parenthood: Influences on pre- and postnatal adjustment in fathers and mothers. J. Psychosom. Obstet. Gynaecol. 2000, 21, 109–120. [Google Scholar] [CrossRef]
- Witt, W.P.; DeLeire, T.; Hagen, E.W.; Wichmann, M.A.; Wisk, L.E.; Spear, H.A.; Cheng, E.R.; Maddox, T.; Hampton, J. The prevalence and determinants of antepartum mental health problems among women in the USA: A nationally representative population-based study. Arch. Women’s Ment. Health 2010, 13, 425–437. [Google Scholar] [CrossRef]
- Hobfoll, S.E.; Ritter, C.; Lavin, J.; Hulsizer, M.R.; Cameron, R.P. Depression prevalence and incidence among inner-city pregnant and postpartum women. J. Consult. Clin. Psychol. 1995, 63, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Kleinman, K.; Abrams, A.; Harlow, B.L.; Mc Laughlin, T.J.; Joffe, H.; Gillman, M.W. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J. Epidemiol. Community Health 2006, 60, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melville, J.L.; Gavin, A.; Guo, Y.; Fan, M.Y.; Katon, W.J. Depressive disorders during pregnancy: Prevalence and risk factors in a large urban sample. Obstet. Gynecol. 2010, 116, 1064–1070. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Whiffen, V.E.; Mount, J.H.; Milne, K.; Cordy, N.I. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J. Consult. Clin. Psychol. 1989, 57, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Guardino, C.M.; Schetter, C.D. Coping during pregnancy: A systematic review and recommendations. Health Psychol. Rev. 2014, 8, 70–94. [Google Scholar] [CrossRef]
- Hobel, C.J.; Goldstein, A.; Barrett, E.S. Psychosocial stress and pregnancy outcome. Clin. Obstet. Gynecol. 2008, 51, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Alderdice, F.; Lynn, F.; Lobel, M. A review and psychometric evaluation of pregnancy-specific stress measures. J. Psychosom. Obstet. Gynaecol. 2012, 33, 62–77. [Google Scholar] [CrossRef]
- Dunkel Schetter, C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annu. Rev. Psychol. 2011, 62, 531–558. [Google Scholar] [CrossRef]
- Anderson, K.G. Establishment of Legal Paternity for Children of Unmarried American Women: Trade-Offs in Male Commitment to Paternal Investment. Hum. Nat. 2017, 28, 168–200. [Google Scholar] [CrossRef]
- Gatny, H.H.; Kusunoki, Y.; Barber, J.S. Pregnancy scares and subsequent unintended pregnancy. Demogr. Res. 2014, 31, 1229–1242. [Google Scholar] [CrossRef] [Green Version]
- Guzzo, K.B.; Hayford, S.R. Unintended fertility and the stability of coresidential relationships. Soc. Sci. Res. 2012, 41, 1138–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.G.; Kaplan, H.; Lancaster, J.B. Demographic correlates of paternity confidence and pregnancy outcomes among Albuquerque men. Am. J. Phys. Anthropol. 2006, 131, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, J.A., Jr.; Jenkins, B.; Rochat, R.W. No fathers’ names: A risk factor for infant mortality in the State of Georgia, USA. Soc. Sci. Med. 1999, 48, 253–265. [Google Scholar] [CrossRef]
- Miller, C.; Garfinkel, I. The determinants of paternity establishment and child support award rates among unmarried women. Popul. Res. Policy Rev. 1999, 18, 237–260. [Google Scholar] [CrossRef]
- Rackin, H.; Gibson-Davis, C.M. The Role of Pre- and Postconception Relationships for First-Time Parents. J. Marriage Fam. Couns. 2012, 74, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Guzzo, K.B. Trends in Cohabitation Outcomes: Compositional Changes and Engagement Among Never-Married Young Adults. J. Marriage Fam. 2014, 76, 826–842. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.S. Paternal factors and low birthweight, preterm, and small for gestational age births: A systematic review. Am. J. Obstet. Gynecol. 2010, 202, 103–123. [Google Scholar] [CrossRef]
- Brown, S.L.; Booth, A. Cohabitation versus Marriage: A comparison of relationship quality. J. Marriage Fam. 1996, 58, 668–678. [Google Scholar] [CrossRef]
- Smock, P.J. Cohabitation in the United States: An Appraisal of Research Themes, Findings, and Implications. Annu. Rev. Sociol. 2000, 26, 1–20. [Google Scholar] [CrossRef]
- Holland, J.A. Love, marriage, then the baby carriage? Marriage timing and childbearing in Sweden. Demogr. Res. 2013, 29, 275–306. [Google Scholar] [CrossRef] [Green Version]
- Glazier, R.H.; Elgar, F.J.; Goel, V.; Holzapfel, S. Stress, social support, and emotional distress in a community sample of pregnant women. J. Psychosom. Obstet. Gynaecol. 2004, 25, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Lavee, Y.; Sharlin, S.; Katz, R. The effect of parenting stress on marital quality: An integrated mother–father model. J. Fam. Issues 1996, 17, 114–135. [Google Scholar] [CrossRef]
- Hildingsson, I.; Tingvall, M.; Rubertsson, C. Partner support in the childbearing period—A follow up study. Women Birth 2008, 21, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lagerberg, D.; Magnusson, M. Utilization of child health services, stress, social support and child characteristics in primiparous and multiparous mothers of 18-month-old children. Scand. J. Public Health 2013, 41, 374–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, H.; Moustafa, M.F.; Amer, H.A.; Hassanein, S.; Keeves, C.; Patel, K. Gestational age, sex and maternal parity correlate with bone turnover in premature infants. Pediatr. Res. 2005, 57 Pt 1, 708–711. [Google Scholar] [CrossRef]
- Masho, S.W.; Chapman, D.; Ashby, M. The impact of paternity and marital status on low birth weight and preterm births. Marriage Fam. Rev. 2010, 46, 243–256. [Google Scholar] [CrossRef]
- Ngui, E.; Cortright, A.; Blair, K. An investigation of paternity status and other factors associated with racial and ethnic disparities in birth outcomes in Milwaukee, Wisconsin. Matern. Child Health J. 2009, 13, 467–478. [Google Scholar] [CrossRef]
- Lewandowski, P.; Bieniek, E. Admitting paternal affiliation with an illegitimate child. Palestra 2012, 7–8, 28–37. (In Polish) [Google Scholar]
- Zeitlin, J.A.; Saurel-Cubizolles, M.J.; Ancel, P.Y. Marital status, cohabitation, and risk of preterm birth in Europe: Where births outside marriage are common and uncommon. Paediatr. Perinat. Epidemiol. 2002, 16, 124–130. [Google Scholar] [CrossRef]
- Hayford, S.R.; Guzzo, K.B.; Smock, P.J. The Decoupling of Marriage and Parenthood? Trends in the Timing of Marital First Births, 1945–2002. J. Marriage Fam. 2014, 76, 520–538. [Google Scholar] [CrossRef]
- Gibson-Davis, C.M.; Ananat, E.O.; Gassman-Pines, A. Midpregnancy Marriage and Divorce: Why the Death of Shotgun Marriage Has Been Greatly Exaggerated. Demography 2016, 53, 1693–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biaggi, A.; Conroy, S.; Pawlby, S.; Pariante, C.M. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J. Affect. Disord. 2016, 191, 62–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raisanen, S.; Lehto, S.M.; Nielsen, H.S.; Gissler, M.; Kramer, M.R.; Heinonen, S. Risk factors for and perinatal outcomes of major depression during pregnancy: A population-based analysis during 2002–2010 in Finland. BMJ Open 2014, 4, e004883. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.M. Depression during pregnancy: Rates, risks and consequences—Motherisk Update 2008. Can. J. Clin. Pharmacol. 2009, 16, e15–e22. [Google Scholar] [PubMed]
- Araya, B.M.; Diaz, M.; Paredes, D.; Ortiz, J. Association between preterm birth and its subtypes and maternal sociodemographic characteristics during the post-transitional phase in a developing country with a very high human development index. Public Health 2017, 147, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Roncarolo, F.; Harper, S. Increasing educational inequality in preterm birth in Quebec, Canada, 1981–2006. J. Epidemiol. Community Health 2011, 65, 1091–1096. [Google Scholar] [CrossRef]
- Braveman, P.A.; Heck, K.; Egerter, S.; Marchi, K.S.; Dominguez, T.P.; Cubbin, C.; Fingar, K.; Pearson, J.A.; Curtis, M. The role of socioeconomic factors in Black-White disparities in preterm birth. Am. J. Public Health 2015, 105, 694–702. [Google Scholar] [CrossRef]
- Blumenshine, P.; Egerter, S.; Barclay, C.J.; Cubbin, C.; Braveman, P.A. Socioeconomic disparities in adverse birth outcomes: A systematic review. Am. J. Prev. Med. 2010, 39, 263–272. [Google Scholar] [CrossRef]
- MacDonald, L.D.; Peacock, J.L.; Anderson, H.R. Marital status: Association with social and economic circumstances, psychological state and outcomes of pregnancy. J. Public Health Med. 1992, 14, 26–34. [Google Scholar]
- Makara-Studzinska, M.; Morylowska-Topolska, J.; Sygit, K.; Sygit, M.; Gozdziewska, M. Socio-demographical and psychosocial determinants of anxiety symptoms in a population of pregnant women in the regions of central and eastern Poland. Ann. Agric. Environ. Med. 2013, 20, 195–202. [Google Scholar]
- Bennett, L.R. Single women’s experiences of premarital pregnancy and induced abortion in Lombok, Eastern Indonesia. Reprod. Health Matters 2001, 9, 37–43. [Google Scholar] [CrossRef]
- Skjeldestad, F.E.; Borgan, J.K.; Daltveit, A.K.; Nymoen, E.H. Induced abortion. Effects of marital status, age and parity on choice of pregnancy termination. Acta Obstet. Gynecol. Scand. 1994, 73, 255–260. [Google Scholar] [CrossRef]
- Wildsmith, E.; Guzzo, K.B.; Hayford, S.R. Repeat unintended, unwanted and seriously mistimed childbearing in the United States. Perspect. Sex. Reprod. Health 2010, 42, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.B. Psychological vulnerability to unwanted pregnancy. Fam. Plan. Perspect. 1973, 5, 199–201. [Google Scholar] [CrossRef]
- Dunkel Schetter, C.; Niles, A.N.; Guardino, C.M.; Khaled, M.; Kramer, M.S. Demographic, Medical, and Psychosocial Predictors of Pregnancy Anxiety. Paediatr. Perinat. Epidemiol. 2016, 30, 421–429. [Google Scholar] [CrossRef]
- Shah, P.S.; Balkhair, T.; Ohlsson, A.; Beyene, J.; Scott, F.; Frick, C. Intention to become pregnant and low birth weight and preterm birth: A systematic review. Matern. Child Health J. 2011, 15, 205–216. [Google Scholar] [CrossRef]
- Martin, L.T.; McNamara, M.J.; Milot, A.S.; Halle, T.; Hair, E.C. The effects of father involvement during pregnancy on receipt of prenatal care and maternal smoking. Matern. Child Health J. 2007, 11, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Elsenbruch, S.; Benson, S.; Rucke, M.; Rose, M.; Dudenhausen, J.; Pincus-Knackstedt, M.K.; Klapp, B.F.; Arck, P.C. Social support during pregnancy: Effects on maternal depressive symptoms, smoking and pregnancy outcome. Hum. Reprod. 2007, 22, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.R.; Hawkins, S.S.; Rifas-Shiman, S.L.; Gillman, M.W.; Taveras, E.M. Association of missing paternal demographics on infant birth certificates with perinatal risk factors for childhood obesity. BMC Public Health 2016, 16, 453. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M.; Figueiredo, B.; Deeds, O.; Contogeorgos, J.; Ascencio, A. Prenatal paternal depression. Infant Behav. Dev. 2006, 29, 579–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Wen, S.W.; Walker, M.; Demissie, K. Missing paternal demographics: A novel indicator for identifying high risk population of adverse pregnancy outcomes. BMC Pregnancy Childbirth 2004, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cai, J. Assessing the exclusionary power of paternity tests involving a pair of alleged parents. Int. J. Legal Med. 2016, 130, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Rini, C.; Dunkel Schetter, C.; Calvin JHobel Glynn, L.M.; Sandman, C.A. Effective social support: Antecedents and consequences of partner support during pregnancy. Pers. Relatsh. 2006, 13, 207–229. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, L.R.; Schetter, C.D.; Westling, E.; Rini, C.; Glynn, L.M.; Hobel, C.J.; Sandman, C.A. Perceived partner support in pregnancy predicts lower maternal and infant distress. J. Fam. Psychol. 2012, 26, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Zuber, M.C. Marital status and cohabitation during pregnancy: Relationship with social conditions, antenatal care and pregnancy outcome in France. Paediatr. Perinat. Epidemiol. 1988, 2, 125–137. [Google Scholar] [CrossRef] [PubMed]
- De Sanjose, S.; Roman, E. Low birthweight, preterm, and small for gestational age babies in Scotland, 1981-1984. J. Epidemiol. Community Health 1991, 45, 207–210. [Google Scholar] [CrossRef]
- Perelli-Harris, B.; Sigle-Rushton, W.; Kreyenfeld, M.; Lappegard, T.; Keizer, R.; Berghammer, C. The educational gradient of childbearing within cohabitation in Europe. Popul. Dev. Rev. 2010, 36, 775–801. [Google Scholar] [CrossRef]
- Perelli-Harris, B.; Styrc, M. Mental Well-Being Differences in Cohabitation and Marriage: The Role of Childhood Selection. J. Marriage Fam. 2018, 80, 239–255. [Google Scholar] [CrossRef]
- Perelli-Harris, B. How Similar are Cohabiting and Married Parents? Second Conception Risks by Union Type in the United States and across Europe. Eur. J. Popul. 2014, 30, 437–464. [Google Scholar] [CrossRef]
- Mynarska, M.; Bernardi, L. Meanings and attitudes attached to cohabitation in Poland: Qualitative analyses of the slow diffusion of cohabitation among the young generation. Demogr. Res. 2007, 16, 519–554. [Google Scholar] [CrossRef]
- Stavrova, O.; Fetchenhauer, D.; Schlosser, T. Why are religious people happy? The effect of the social norm of religiosity across countries. Soc. Sci. Res. 2013, 42, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Stavrova, O.; Fetchenhauer, D.; Schlösser, T. Cohabitation, gender, and happiness: A cross-cultural study in thirty countries. J. Cross-Cult. Psychol. 2012, 43, 1063–1081. [Google Scholar] [CrossRef]
- Bakert, K.K. Bargaining of Biology? The history and future of paternity. Law and parental status. Cornell J. Law Public Policy 2004, 14, 1–69. [Google Scholar]
- Aktan, N.M. Social support and anxiety in pregnant and postpartum women: A secondary analysis. Clin. Nurs. Res. 2012, 21, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.C.; Wilkins, R.; Kramer, M.S. Disparities in pregnancy outcomes according to marital and cohabitation status. Obstet. Gynecol. 2004, 103, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Raatikainen, K.; Heiskanen, N.; Heinonen, S. Marriage still protects pregnancy. Bjog 2005, 112, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Lichter, D.T.; Qian, Z.; Mellott, L.M. Marriage or dissolution? Union transitions among poor cohabiting women. Demography 2006, 43, 223–240. [Google Scholar] [CrossRef]
- Lanier, P.; Jonson-Reid, M. Comparing primiparous and multiparous mothers in a nurse home visiting prevention program. Birth 2014, 41, 344–352. [Google Scholar] [CrossRef]
- Bai, J.; Wong, F.W.; Bauman, A.; Mohsin, M. Parity and pregnancy outcomes. Am. J. Obstet. Gynecol. 2002, 186, 274–278. [Google Scholar] [CrossRef]
- Majoko, F.M.; Nystrom, L.; Munjanja, S.P.; Mason, E.; Lindmark, G. Relation of parity to pregnancy outcome in a rural community in Zimbabwe. Afr. J. Reprod. Health 2004, 8, 198–206. [Google Scholar] [CrossRef]
- Shah, P.S. Parity and low birth weight and preterm birth: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2010, 89, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, L.J.; Schoendorf, K.C.; Kiely, J.L. Risk of preterm birth in multiparous teenagers. Arch. Pediatr. Adolesc. Med. 2000, 154, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.D.; Bushnik, T.; Sheppard, A.J.; Kramer, M.S.; Kaufman, J.S.; Yang, S. Paternal education and adverse birth outcomes in Canada. J. Epidemiol. Community Health 2017, 71, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Capron, L.E.; Ramchandani, P.G.; Glover, V. Maternal prenatal stress and placental gene expression of NR3C1 and HSD11B2: The effects of maternal ethnicity. Psychoneuroendocrinology 2018, 87, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Davis, C.; Gassman-Pines, A.; Lehrman, R. “His” and “Hers”: Meeting the Economic Bar to Marriage. Demography 2018, 55, 2321–2343. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Sassler, S.; Frech, A.; Addo, F.; Cooksey, E. Nonmarital Childbearing, Union History, and Women’s Health at Midlife. Am. Sociol. Rev. 2011, 76, 465–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bublitz, M.H.; Vergara-Lopez, C.; O’Reilly Treter, M.; Stroud, L.R. Association of Lower Socioeconomic Position in Pregnancy with Lower Diurnal Cortisol Production and Lower Birthweight in Male Infants. Clin. Ther. 2016, 38, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Challis, J.R.; Smith, S.K. Fetal endocrine signals and preterm labor. Biol. Neonate 2001, 79, 163–167. [Google Scholar]
- Talge, N.M.; Neal, C.; Glover, V. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J. Child Psychol. Psychiatry 2007, 48, 245–261. [Google Scholar] [CrossRef]
- Desantis, A.S.; Kuzawa, C.W.; Adam, E.K. Developmental origins of flatter cortisol rhythms: Socioeconomic status and adult cortisol activity. Am. J. Hum. Biol. 2015, 27, 458–467. [Google Scholar] [CrossRef]
- Gilles, M.; Otto, H.; Wolf, I.A.C.; Scharnholz, B.; Peus, V.; Schredl, M.; Sutterlin, M.W.; Witt, S.H.; Rietschel, M.; Laucht, M.; et al. Maternal hypothalamus-pituitary-adrenal (HPA) system activity and stress during pregnancy: Effects on gestational age and infant’s anthropometric measures at birth. Psychoneuroendocrinology 2018, 94, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Rakers, F.; Rupprecht, S.; Dreiling, M.; Bergmeier, C.; Witte, O.W.; Schwab, M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2017. [CrossRef] [PubMed]
- Bleker, L.S.; Roseboom, T.J.; Vrijkotte, T.G.; Reynolds, R.M.; de Rooij, S.R. Determinants of cortisol during pregnancy—The ABCD cohort. Psychoneuroendocrinology 2017, 83, 172–181. [Google Scholar] [CrossRef]
- Beijers, R.; Buitelaar, J.K.; de Weerth, C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: Beyond the HPA axis. Eur. Child Adolesc. Psychiatry 2014, 23, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.P.; Leader, L. Maternal stress and obstetric and infant outcomes: Epidemiological findings and neuroendocrine mechanisms. Aust. N. Z. J. Obstet. Gynaecol. 2000, 40, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Habersaat, S.; Borghini, A.; Nessi, J.; Forcada-Guex, M.; Muller-Nix, C.; Pierrehumbert, B.; Ansermet, F. Effects of perinatal stress and maternal traumatic stress on the cortisol regulation of preterm infants. J Trauma Stress 2014, 27, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.A.; Wadhwa, P.D.; Chicz-DeMet, A.; Dunkel-Schetter, C.; Porto, M. Maternal stress, HPA activity, and fetal/infant outcome. Ann. N. Y. Acad. Sci. 1997, 814, 266–275. [Google Scholar] [CrossRef]
- Mancuso, R.A.; Schetter, C.D.; Rini, C.M.; Roesch, S.C.; Hobel, C.J. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom. Med. 2004, 66, 762–769. [Google Scholar] [CrossRef]
- Garcia-Blanco, A.; Diago, V.; Serrano De La Cruz, V.; Hervas, D.; Chafer-Pericas, C.; Vento, M. Can stress biomarkers predict preterm birth in women with threatened preterm labor? Psychoneuroendocrinology 2017, 83, 19–24. [Google Scholar] [CrossRef]
- Guardino, C.M.; Schetter, C.D.; Saxbe, D.E.; Adam, E.K.; Ramey, S.L.; Shalowitz, M.U. Diurnal salivary cortisol patterns prior to pregnancy predict infant birth weight. Health Psychol. 2016, 35, 625–633. [Google Scholar] [CrossRef]
- Bolten, M.I.; Wurmser, H.; Buske-Kirschbaum, A.; Papousek, M.; Pirke, K.M.; Hellhammer, D. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Arch. Women’s Ment. Health 2011, 14, 33–41. [Google Scholar] [CrossRef]
- Bracero, L.; Broce, M.; Kali, M.; Nguyen, M.; Reyes, B. The investigation of missing paternal information in birth certificates and low birth weight. J. Matern. Fetal Neonatal Med. 2009, 22, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.R.; Patterson, S.; Beattie, B. Single-parent households and children’s educational achievement: A state-level analysis. Soc. Sci. Res. 2015, 53, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichman, N.E.; Corman, H.; Noonan, K. Effects of child health on parents’ relationship status. Demography 2004, 41, 569–584. [Google Scholar] [CrossRef]
- Huizink, A.C.; Mulder, E.J.; Robles de Medina, P.G.; Visser, G.H.; Buitelaar, J.K. Is pregnancy anxiety a distinctive syndrome? Early Hum. Dev. 2004, 79, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Straub, H.; Qadir, S.; Miller, G.; Borders, A. Stress and stress reduction. Clin. Obstet. Gynecol. 2014, 57, 579–606. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Estimate/Level | Term Birth | Preterm Birth | Risk Ratio (RR) | −95% CI | +95% CI |
|---|---|---|---|---|---|---|
| Maternal age (cont.) | n | 83,610 | 4306 | 1.02 | 1.02 | 1.03 |
| mean | 28.1 | 28.7 | ||||
| std. dev. | 5.2 | 6.0 | ||||
| Sex of the infant | Boy | 42,907 | 2353 | 1.14 | 1.07 | 1.20 |
| 51.3% | 54.6% | |||||
| Girl | 40,703 | 1953 | ||||
| 48.7% | 45.4% | |||||
| Parity | Multiparous | 37,973 | 2052 | 1.09 | 1.03 | 1.16 |
| 45.4% | 47.7% | |||||
| Primiparous | 45,632 | 2253 | ||||
| 54.6% | 52.3% | |||||
| Maternal marital status | Unmarried | 11,811 | 915 | 1.60 | 1.49 | 1.71 |
| 14.1% | 21.2% | |||||
| Married | 71,799 | 3391 | ||||
| 85.9% | 78.8% | |||||
| Maternal employment | Unemployed | 21,414 | 1330 | 1.29 | 1.21 | 1.37 |
| 25.7% | 31.1% | |||||
| Employed | 61,918 | 2946 | ||||
| 74.3% | 68.9% | |||||
| Maternal education | Lower | 18,365 | 1298 | 1.54 | 1.42 | 1.61 |
| 22.0% | 30.3% | |||||
| Higher | 64,967 | 2979 | ||||
| 78.0% | 69.7% | |||||
| Emp & Edu Mother | NE-LE | 8718 | 711 | 1.75 | 1.61 | 1.90 |
| 10.5% | 16.6% | |||||
| E-LE | 9628 | 585 | 1.33 | 1.21 | 1.45 | |
| 11.6% | 13.7% | |||||
| NE-HE | 12,679 | 617 | 1.07 | 0.98 | 1.17 | |
| 15.2% | 14.4% | |||||
| E-HE | 52,253 | 2360 | ||||
| 62.7% | 55.2% | |||||
| Marital-Father Data index | UM-FDA | 3449 | 357 | 2.07 | 1.86 | 2.30 |
| 4.1% | 8.3% | |||||
| UM-FDP | 8362 | 558 | 1.37 | 1.26 | 1.50 | |
| 10.0% | 13.0% | |||||
| MAC-FDP | 15,101 | 688 | 0.96 | 0.88 | 1.04 | |
| 18.1% | 16.0% | |||||
| MBC-FDP | 56,698 | 2703 | ||||
| 67.8% | 62.8% |
| Characteristics | Estimate/Level | Primiparous | Multiparous | p | ANOVA/Pearson Chi-square |
|---|---|---|---|---|---|
| Maternal age (cont.) | n | 47,885 | 40,025 | <0.01 | F (1, 87,908) = 19,578.0 |
| mean | 26.1 | 30.6 | |||
| std.dev. | 4.6 | 4.8 | |||
| Sex of the infant | Boy | 24,818 | 20,438 | 0.02 | Pearson Chi-square: 5.1; df = 1 |
| 51.8% | 51.1% | ||||
| Girl | 23,067 | 19,587 | |||
| 48.2% | 48.9% | ||||
| Maternal marital status | Unmarried | 8478 | 4244 | <0.01 | Pearson Chi-square: 888.3; df = 1 |
| 17.7% | 10.6% | ||||
| Married | 39,407 | 35,781 | |||
| 82.3% | 89.4% | ||||
| Maternal employment | Unemployed | 12,007 | 10,737 | <0.01 | Pearson Chi-square: 37.7; df = 1 |
| 25.1% | 27.0% | ||||
| Employed | 35,770 | 29,090 | |||
| 74.9% | 73.0% | ||||
| Maternal education | Lower | 9122 | 10,540 | <0.01 | Pearson Chi-square: 677.0; df = 1 |
| 19.1% | 26.5% | ||||
| Higher | 38,650 | 29,293 | |||
| 80.9% | 73.5% | ||||
| Emp & Edu Mother | NE-LE | 4437 | 4992 | <0.01 | Pearson Chi-square: 676.9; df = 3 |
| 9.3% | 12.5% | ||||
| E-LE | 4678 | 5534 | |||
| 9.8% | 13.9% | ||||
| NE-HE | 7558 | 5738 | |||
| 15.8% | 14.4% | ||||
| E-HE | 31,067 | 23,543 | |||
| 65.1% | 59.1% | ||||
| Birth status | TB | 45,632 | 37,973 | <0.01 | Pearson Chi-square: 8,3, df = 1 |
| 95.3% | 94.9% | ||||
| PTB | 2253 | 2052 | |||
| 4.7% | 5.1% |
| Characteristics | Estimate/Level | Marital-Father Data Index | p | ANOVA/Pearson Chi-square | |||
|---|---|---|---|---|---|---|---|
| MBC-FDP | MAC-FDP | UM-FDP | UM-FDA | ||||
| Maternal age cont.) | n | 59,401 | 15,789 | 8920 | 3806 | <0.01 | F(3; 87,912) = 5511.1 |
| mean | 29.5 | 24.7 | 26.8 | 23.9 | |||
| std.dev. | 4.48 | 4.58 | 6.08 | 6.45 | |||
| Sex of the infant | boy | 30,519 | 8133 | 4625 | 1983 | 0.72 | Pearson Chi-square: 1.33; df = 3 |
| 51.4% | 51.5% | 51.8% | 52.1% | ||||
| girl | 28,882 | 7656 | 4295 | 1823 | |||
| 48.6% | 48.5% | 48.2% | 47.9% | ||||
| Parity | multiparous | 34,478 | 1303 | 3126 | 1118 | <0.01 | Pearson Chi-square: 13,392.3; df = 3 |
| 58.0% | 8.3% | 35.0% | 29.4% | ||||
| primiparous | 24,921 | 14,486 | 5793 | 2685 | |||
| 42.0% | 91.7% | 65.0% | 70.6% | ||||
| Maternal marital status | unmarried | 0 | 0 | 8920 | 3806 | <0.01 | Pearson Chi-square: 87,916.0; df = 3 |
| 0.0% | 0.0% | 100.0% | 100.0% | ||||
| married | 59,401 | 15,789 | 0 | 0 | |||
| 100.0% | 100.0% | 0.0% | 0.0% | ||||
| Maternal employment | unemployed | 11,795 | 5280 | 3400 | 2269 | <0.01 | Pearson Chi-square: 4770.8; df = 3 |
| 19.9% | 33.5% | 38.2% | 62.0% | ||||
| employed | 47,489 | 10,496 | 5490 | 1389 | |||
| 80.1% | 66.5% | 61.8% | 38.0% | ||||
| Maternal education | lower | 10,166 | 3909 | 3272 | 2316 | <0.01 | Pearson Chi-square: 5575.9; df = 3 |
| 17.1% | 24.8% | 36.8% | 63.4% | ||||
| higher | 49,131 | 11,857 | 5619 | 1339 | |||
| 82.9% | 75.2% | 63.2% | 36.6% | ||||
| Emp & Edu Mother | NE-LE | 3943 | 1779 | 1953 | 1754 | <0.01 | Pearson Chi-square: 9565.03; df = 9 |
| 6.7% | 11.3% | 22.0% | 48.1% | ||||
| E-LE | 6218 | 2129 | 1311 | 555 | |||
| 10.5% | 13.5% | 14.8% | 15.2% | ||||
| NE-HE | 7847 | 3497 | 1442 | 510 | |||
| 13.2% | 22.2% | 16.2% | 14.0% | ||||
| E-HE | 41,258 | 8355 | 4172 | 828 | |||
| 69.6% | 53.0% | 47.0% | 22.7% | ||||
| Marital-Father Data Index | RR | RR−95% CI | RR+95% CI | RR | RR−95% CI | RR+95% CI | ||
|---|---|---|---|---|---|---|---|---|
| All | Crude | Adjusted * | ||||||
| MBC-FDP | [ref.cat.] | 1.00 | 1.00 | |||||
| MAC-FDP | 0.96 | 0.88 | 1.04 | 1.06 | 0.97 | 1.17 | ||
| UM-FDP | 1.37 | 1.26 | 1.50 | 1.33 | 1.22 | 1.46 | ||
| UM-FDA | 2.07 | 1.86 | 2.30 | 1.93 | 1.71 | 2.17 | ||
| p for trend < 0.001 # | ||||||||
| Primiparous | Crude | Adjusted ** | ||||||
| MBC-FDP | [ref.cat.] | 1.00 | 1.00 | |||||
| MAC-FDP | 0.93 | 0.85 | 1.03 | 1.00 | 0.90 | 1.11 | ||
| UM-FDP | 1.17 | 1.04 | 1.33 | 1.20 | 1.05 | 1.36 | ||
| UM-FDA | 1.78 | 1.54 | 2.04 | 1.83 | 1.57 | 2.13 | ||
| p for trend < 0.001 # | ||||||||
| Multiparous | Crude | Adjusted ** | ||||||
| MBC-FDP | [ref.cat.] | 1.00 | 1.00 | |||||
| MAC-FDP | 1.32 | 1.06 | 1.64 | 1.29 | 1.03 | 1.60 | ||
| UM-FDP | 1.75 | 1.54 | 1.99 | 1.51 | 1.33 | 1.72 | ||
| UM-FDA | 2.80 | 2.39 | 3.29 | 2.16 | 1.81 | 2.58 | ||
| p for trend < 0.001 # | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merklinger-Gruchala, A.; Kapiszewska, M. The Effect of Prenatal Stress, Proxied by Marital and Paternity Status, on the Risk of Preterm Birth. Int. J. Environ. Res. Public Health 2019, 16, 273. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16020273
Merklinger-Gruchala A, Kapiszewska M. The Effect of Prenatal Stress, Proxied by Marital and Paternity Status, on the Risk of Preterm Birth. International Journal of Environmental Research and Public Health. 2019; 16(2):273. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16020273
Chicago/Turabian StyleMerklinger-Gruchala, Anna, and Maria Kapiszewska. 2019. "The Effect of Prenatal Stress, Proxied by Marital and Paternity Status, on the Risk of Preterm Birth" International Journal of Environmental Research and Public Health 16, no. 2: 273. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16020273