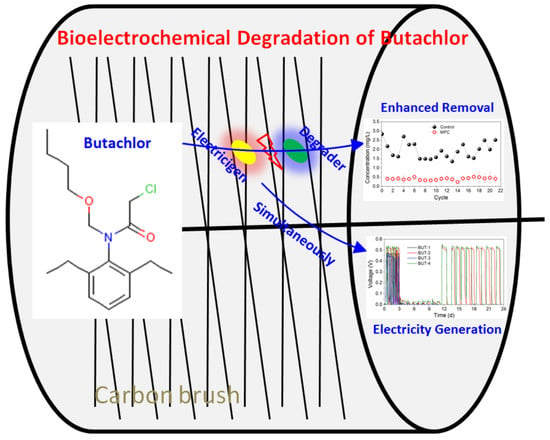
Efficient Removal of Butachlor and Change in Microbial Community Structure in Single-Chamber Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Construction and Operation
2.2. Addition of Butachlor and MFC Acclimation
2.3. Measurement of Butachlor
2.4. Microbial Community Analysis
3. Results
3.1. Butachlor Removal and Electricity Generation
3.2. Microbial Community Richness and Diversity Indices
3.3. Change in Microbial Abundances at the Class Level
3.4. Change in Microbial Abundances at the Genus Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.E.; Hladik, M.L.; Kolpin, D.W. Occurrence of dichloroacetamide herbicide safeners and co-applied herbicides in midwestern U.S. streams. Environ. Sci. Technol. Lett. 2018, 5, 3–8. [Google Scholar] [CrossRef]
- Mccarroll, N.E.; Stack, H.F.F.; Protzel, A.; Ioannou, Y.; Jackson, M.A.; Waters, M.D.; Dearfield, K.L. A survey of EPA/OPP and open literature on selected pesticide chemicals: III. Mutagenicity and carcinogenicity of benomyl and carbendazim. Mutat Res. 2002, 512, 1–35. [Google Scholar] [CrossRef]
- Coleman, S.; Linderman, R.; Hodgson, E.; Rose, R.L. Comparative metabolism of chloroacetamide herbicides and selected metabolites in human and rat liver microsomes. Environ. Health Persp. 2000, 108, 1151–1157. [Google Scholar]
- Xu, G.M.; Zheng, Y.Y.; Wang, S.H.; Zhang, J.S.; Yan, Y.C. Biodegradation of chlorpyrifos and 3,5,6-trichloro-2-pyridinol by a newly isolated Paracoccus sp. strain TRP. Int. Biodeter. Biodegr. 2008, 62, 51–56. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, J.W.; Liang, B.; Wang, C.H.; Cai, S.; Ni, Y.Y.; He, J.; Li, S.P. Biodegradation of chloroacetamide herbicides by Paracoccus sp. FLY-8 in vitro. J. Agric. Food Chem. 2011, 59, 4614–4621. [Google Scholar] [CrossRef] [PubMed]
- Accinelli, C.; Dinelli, G.; Vicari, A.; Catizone, P. Atrazine and metolachlor degradation in subsoils. Biol. Fert. Soils 2001, 33, 495–500. [Google Scholar] [CrossRef]
- Bedmar, F.; Gimenez, D.; Costa, J.L.; Daniel, P.E. Persistence of acetochlor, atrazine and S-metolachlor in surface and sub-surface horizons of two typic argiudolls under no-tillage. Environ. Toxicol. Chem. 2017, 36, 3065–3073. [Google Scholar] [CrossRef]
- Friedman, C.L.; And, A.T.L.; Hay, A. Degradation of chloroacetanilide herbicides by anodic fenton treatment. J. Agric. Food Chem. 2006, 54, 2640–2651. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Rittmann, B.E. Chemistry: Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Slade, R.C.T.; Varcoe, J.R. Techniques for the study and development of microbial fuel cells: An electrochemical perspective. Chem. Soc. Rev. 2009, 38, 1926–1939. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, Y.; Ding, N.; Zhou, Q. Opening size optimization of metal matrix in rolling-pressed activated carbon air–cathode for microbial fuel cells. Appl. Energ. 2014, 123, 13–18. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, Y.; Gao, N.; Li, D.; Zhou, Q. Effects of catalyst layer and gas diffusion layer thickness on the performance of activated carbon air-cathode for microbial fuel cells. Int. J. Electrochem. Sci. 2015, 10, 5086–5100. [Google Scholar]
- Zhang, Y.; Wang, X.; Li, X.; Gao, N.; Wan, L.; Feng, C.; Zhou, Q. A novel and high performance activated carbon air-cathode with decreased volume density and catalyst layer invasion for microbial fuel cells. RSC Adv. 2014, 4, 42577–42580. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar]
- Wan, Y.; Zhou, L.; Wang, S.; Liao, C.; Li, N.; Liu, W.; Wang, X. Syntrophic growth of Geobacter sulfurreducens accelerates anaerobic denitrification. Front. Microbiol. 2018, 9, 1572. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Schleinitz, K.M.; Schmeling, S.; Jehmlich, N.; Bergen, M.V.; Harms, H.; Kleinsteuber, S.; Vogt, C.; Fuchs, G. Phenol degradation in the strictly anaerobic iron-reducing bacterium Geobacter metallireducens GS-15. Appl. Environ. Microb. 2009, 75, 3912. [Google Scholar] [CrossRef]
- Wischgoll, S.; Heintz, D.; Peters, F.; Erxleben, A.; Sarnighausen, E.; Reski, R.; Dorsselaer, A.V.; Boll, M. Gene clusters involved in anaerobic benzoate degradation of Geobacter metallireducens. Mol. Microbiol. 2005, 58, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tremblay, P.L.; Chaurasia, A.K.; Smith, J.A.; Bain, T.S.; Lovley, D.R. Anaerobic benzene oxidation via phenol in Geobacter metallireducens. Appl. Environ. Microb. 2013, 79, 7800–7806. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, B.L.; Sayavedra-Soto, L.A.; Bottomley, P.J.; Arp, D.J. Thauera butanivorans sp. nov., a C2-C9 alkane-oxidizing bacterium previously referred to as ‘Pseudomonas butanovora’. Int. J. Syst. Evol. Microbiol. 2009, 59, 1576. [Google Scholar] [CrossRef] [PubMed]
- Cooley, R.B.; Dubbels, B.L.; Sayavedra-Soto, L.A.; Bottomley, P.J.; Arp, D.J. Kinetic characterization of the soluble butane monooxygenase from Thauera butanivorans, formerly ‘Pseudomonas butanovora’. Microbiology 2009, 155, 2086. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, Y.; Zhang, T. Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour. Technol. 2013, 128, 703–710. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, Y.; Zhao, L.; Sun, Y.; Zhang, X.; Chen, X.; Weng, L.; Li, Y. Efficient Removal of Butachlor and Change in Microbial Community Structure in Single-Chamber Microbial Fuel Cells. Int. J. Environ. Res. Public Health 2019, 16, 3897. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16203897
Li X, Li Y, Zhao L, Sun Y, Zhang X, Chen X, Weng L, Li Y. Efficient Removal of Butachlor and Change in Microbial Community Structure in Single-Chamber Microbial Fuel Cells. International Journal of Environmental Research and Public Health. 2019; 16(20):3897. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16203897
Chicago/Turabian StyleLi, Xiaojing, Yue Li, Lixia Zhao, Yang Sun, Xiaolin Zhang, Xiaodong Chen, Liping Weng, and Yongtao Li. 2019. "Efficient Removal of Butachlor and Change in Microbial Community Structure in Single-Chamber Microbial Fuel Cells" International Journal of Environmental Research and Public Health 16, no. 20: 3897. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16203897