The Association Between Cephalosporin and Hypoprothrombinemia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
3. Results
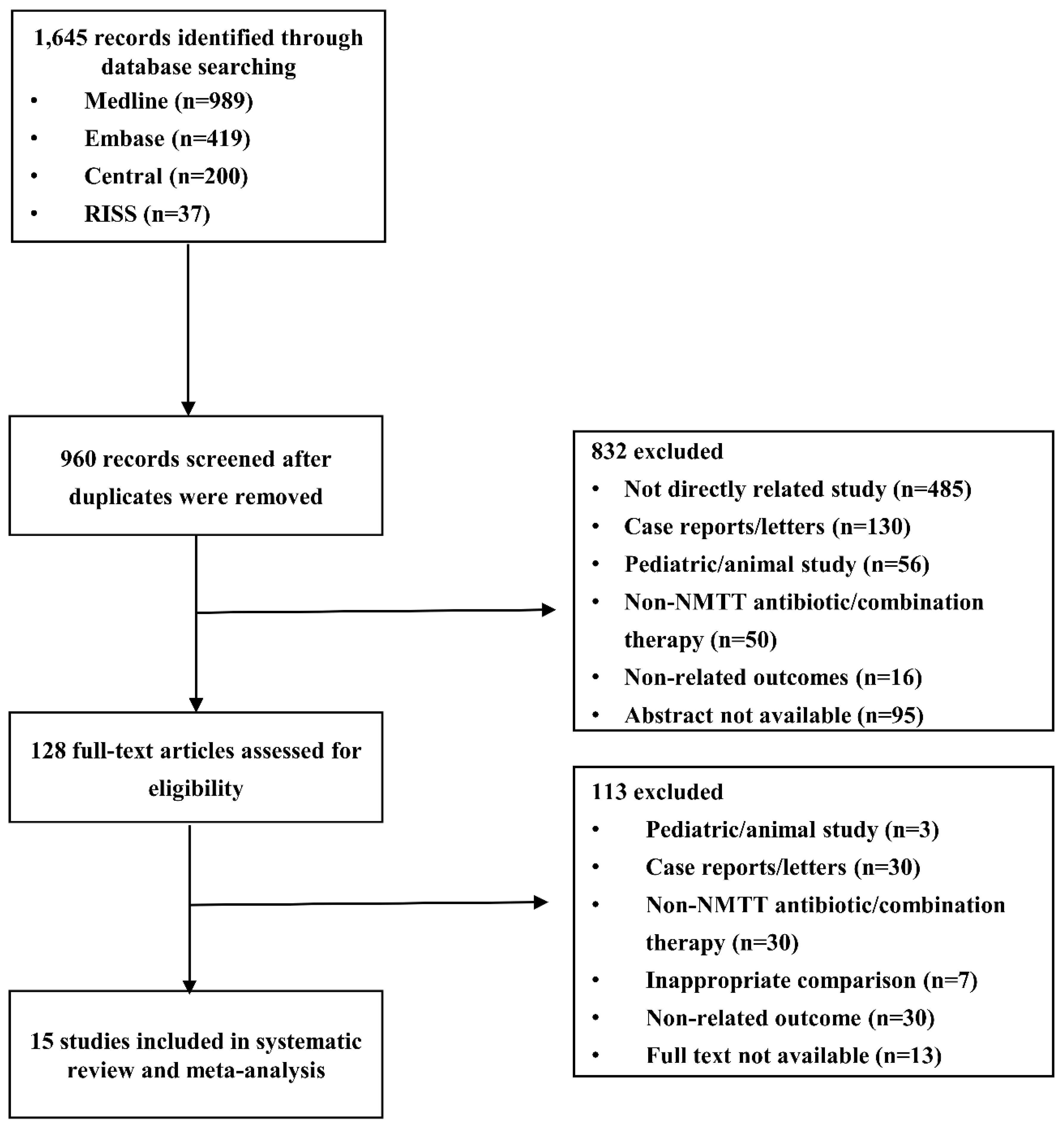
3.1. Literature Search
3.2. Study Characteristics and Quality
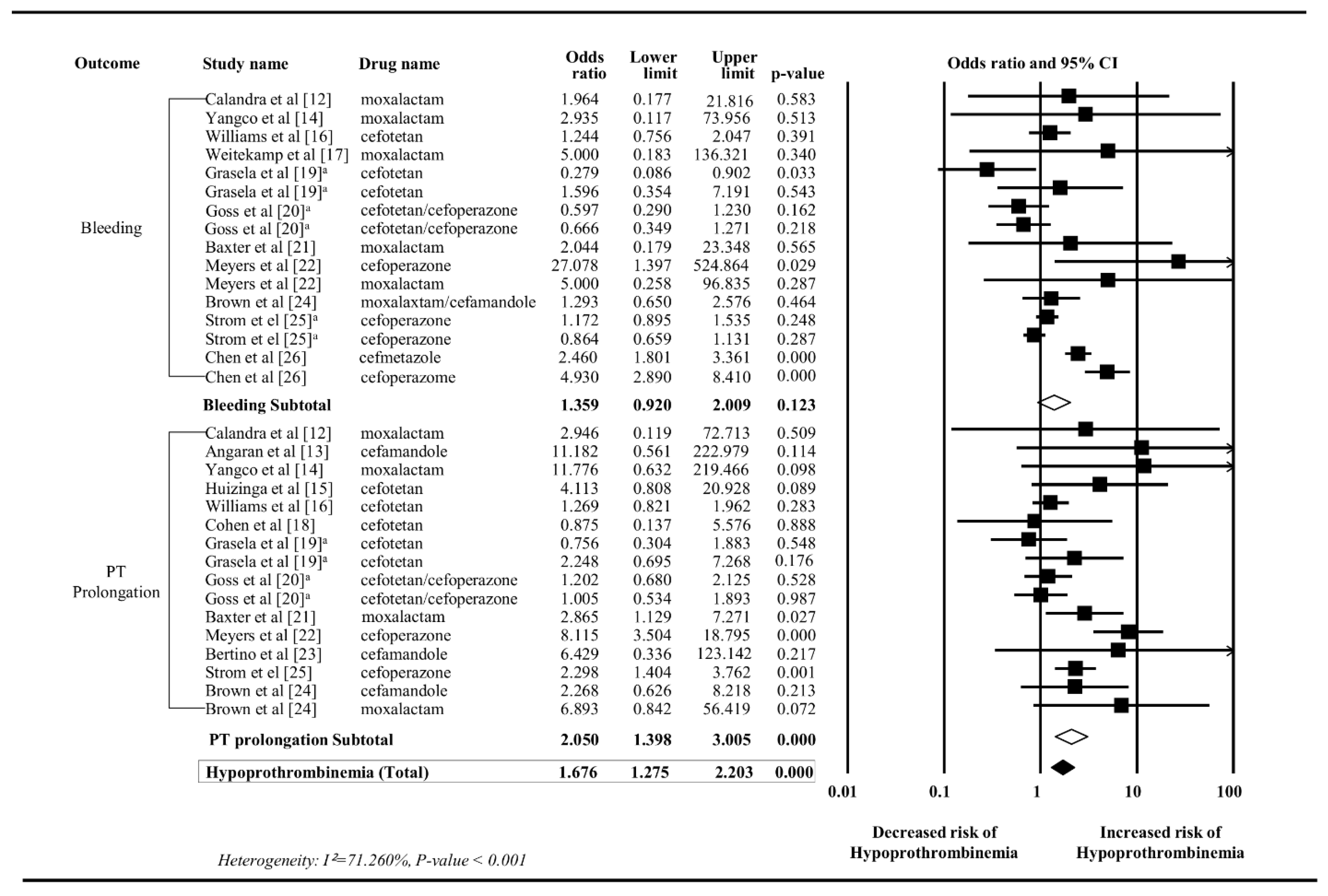
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eom, J.S. Investigation for Proper Utilization of Cephalosporins in Outpatient Clinics; Final Report; Korea Centers for Disease Control & Prevention: Osong, Cheongju, Korea, 2011. (In Korean) [Google Scholar]
- Ministry of Food and Drug Safety. Available online: https://nedrug.mfds.go.kr/index (accessed on 13 August 2019).
- U.S. Food & Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=050588 (accessed on 13 August 2019).
- Chambers, H.F. Beta-lactam & other cell wall- & membrane-active antibiotics. In Basic and Clinical Pharmacology, 10th ed.; Katzung, B.G., Ed.; McGraw-Hill: New York, NY, USA, 2007; pp. 726–744. [Google Scholar]
- Wold, J.S.; Buening, M.K.; Hanasono, G.K. Latamoxef-associated hypoprothrombinaemia. Lancet 1983, 2, 408. [Google Scholar] [CrossRef]
- Shearer, M.J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P.T.; Trenk, D.; Jähnchen, E.; Ritz, E. Mechanism of cephalosporin-induced hypoprothrombinemia: Relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J. Clin. Pharmacol. 1988, 28, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, Y.M.; Conly, J.M. Antibiotic-associated hypoprothrombinemia: A review of prospective studies, 1966–1988. Rev. Infect. Dis. 1990, 12, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. (accessed on 19 July 2019).
- Hagstrom, J.N.; Harper, J.L. Medscape. Available online: https://emedicine.medscape.com/article/956030-clinical (accessed on 19 July 2019).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Independent subgroups within a study. In Introduction to Meta-Analysis; Sharples, K., Woodward, G., Barclay, S., Dufour, B., Kay, H., Jayachandran, S., Eds.; John Wiley & Sons: New Jersey, NJ, USA, 2009; pp. 217–223. [Google Scholar]
- Calandra, G.B.; Hesney, M.; Grad, C. A multiclinic randomized study of the comparative efficacy, safety and tolerance of imipenem/cilastatin and moxalactam. Eur. J. Clin. Microbiol. 1984, 3, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Angaran, D.M.; Dias, V.C.; Arom, K.V.; Northrup, W.F.; Kersten, T.G.; Lindsay, W.G.; Nicoloff, D.M. The comparative influence of prophylactic antibiotics on the prothrombin response to warfarin in the postoperative prosthetic cardiac valve patient. Cefamandole, cefazolin, vancomycin. Ann. Surg. 1987, 206, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yangco, B.G.; Baird, I.; Lorber, B.; Noble, R.; Bermudez, R.; Silverblatt, F.; Vasquez, G. Comparative efficacy and safety of ceftizoxime, cefotaxime and latamoxef in the treatment of bacterial pneumonia in high risk patients. J. Antimicrob. Chemother. 1987, 19, 239–248. [Google Scholar] [CrossRef]
- Huizinga, W.K.J.; Baker, L.W.; Kadwa, H.; Van Den Ende, J.; Francis, A.J.; Francis, G.M. Management of severe intraabdominal sepsis: Single agent antibiotic therapy with cefotetan versus combination therapy with ampicillin, gentamicin and metronidazole. Br. J. Surg. 1988, 75, 1134–1138. [Google Scholar] [CrossRef]
- Williams, K.J.; Bax, R.P.; Brown, H.; Machin, S.J. Antibiotic treatment and associated prolonged prothrombin time. J. Clin. Pathol. 1991, 44, 738–741. [Google Scholar] [CrossRef]
- Weitekamp, M.R.; Caputo, G.M.; Al-Mondhiry, H.A.; Aber, R.C. The effects of latamoxef, cefotaxime, and cefoperazone on platelet function and coagulation in normal volunteers. J. Antimicrob. Chemother. 1985, 16, 95–101. [Google Scholar] [CrossRef]
- Cohen, H.; Scott, S.D.; Mackie, I.J.; Shearer, M.; Bax, R.; Karran, S.J.; Machin, S.J. The development of hypoprothrombinaemia following antibiotic therapy in malnourished patients with low serum vitamin K1 levels. Br. J. Haematol. 1988, 68, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Grasela, T.H., Jr.; Walawander, C.A.; Welage, L.S.; Wing, P.E.; Scarafoni, D.J.; Caldwell, J.W.; Noguchi, J.K.; Schentag, J.J. Prospective surveillance of antibiotic-associated coagulopathy in 970 patients. Pharmacotherapy 1989, 9, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Goss, T.F.; Walawander, C.A.; Grasela, T.H., Jr.; Meisel, S.; Katona, B.; Jaynes, K. Prospective evaluation of risk factors for antibiotic-associated bleeding in critically ill patients. Pharmacotherapy 1992, 12, 283–291. [Google Scholar] [PubMed]
- Baxter, J.G.; Marble, D.A.; Whitfield, L.R.; Wels, P.B.; Walczak, P.; Schentag, J.J. Clinical risk factors for prolonged PT/PTT in abdominal sepsis patients treated with moxalactam or tobramycin plus clindamycin. Ann. Surg. 1985, 201, 96–102. [Google Scholar] [PubMed]
- Meyers, B.R. Comparative toxicities of third-generation cephalosporins. Am. J. Med. 1985, 79, 96–103. [Google Scholar] [CrossRef]
- Bertino, J.S., Jr.; Kozak, A.J.; Reese, R.E.; Chiarello, L.A. Hypoprothrombinemia associated with cefamandole use in a rural teaching hospital. Arch. Intern. Med. 1986, 146, 1125–1128. [Google Scholar] [CrossRef]
- Brown, R.B.; Klar, J.; Lemeshow, S.; Teres, D.; Pastides, H.; Sands, M. Enhanced bleeding with cefoxitin or moxalactam. Statistical analysis within a defined population of 1493 patients. Arch. Intern. Med. 1986, 146, 2159–2164. [Google Scholar] [CrossRef]
- Strom, B.L.; Schinnar, R.; Gibson, G.A.; Brennan, P.J.; Berlin, J.A. Risk of bleeding and hypoprothrombinaemia associated with NMTT side chain antibiotics: Using cefoperazone as a test case. Pharmacoepidemiol. Drug Saf. 1999, 8, 81–94. [Google Scholar] [CrossRef]
- Chen, L.J.; Hsiao, F.Y.; Shen, L.J.; Wu, F.L.L.; Tsay, W.; Hung, C.C.; Lin, S.W. Use of hypoprothrombinemia-inducing cephalosporins and the risk of hemorrhagic events: A nationwide nested case-control study. PLoS ONE 2016, 11, e0158407. [Google Scholar] [CrossRef]
- Kamal, A.H.; Tefferi, A.; Pruthi, R.K. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin. Proc. 2007, 82, 864–873. [Google Scholar] [CrossRef]
- Welage, L.S.; Hejmanowski, L.G.; Wilton, J.H.; Walawander, C.; Rigan, D.; Williams, J.S.; Schentag, J.J. Comparison of N-methylthiotetrazole dispositions in healthy volunteers following single intravenous doses of moxalactam, cefoperazone, and cefotetan. Antimicrob. Agents Chemother. 1989, 33, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agenzia Italiana del Farmaco (AIFA). Available online: https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/home (accessed on 13 August 2019).
- Compendium. Available online: https://compendium.ch/home/de?Platform=Desktop (accessed on 13 August 2019).
- Base de Donnees Publique des Medicaments. Available online: http://base-donnees-publique.medicaments.gouv.fr/ (accessed on 13 August 2019).
- Pharmaceuticals and Medical Devices Agency. Available online: https://www.pmda.go.jp/english/ (accessed on 13 August 2019).
- Jones, P.G.; Strother, S.V.; Rolston, K.V.I. Hypoprothrombinemia in patients with cancer receiving cefoperazone and mezlocillin. Arch. Intern. Med. 1986, 146, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.R. Fatal vitamin K-dependent coagulopathy associated with cefoperazone/sulbactam: A case report. Drug Saf. Case Rep. 2019, 6, 6. [Google Scholar] [CrossRef]
- Nakano, E.; Fukuoka, T.; Takeuchi, N.; Seki, T.; Tamai, M.; Araki, M. A Case of alveolar bleeding from clotting abnormality by cefmetazole. Case Rep. Med. 2019, 3574064. [Google Scholar] [CrossRef]


| Study | Country | Study Design | Population Characteristics | Total Population | Intervention a | Control | Outcomes and Definitions | QA Score c | |
|---|---|---|---|---|---|---|---|---|---|
| PT b | Bleeding | ||||||||
| Calandra et al. (1984) [12] | United States | RCT | Infections | 441 | Moxalactam 2g q8h for 5–14 days | Imipenem/cilastatin | PT increases by 3s or 25% | Hemorrhagic colitis, hemoperitoneum, GI hemorrhage | – |
| Angaran et al. (1987) [13] | United States | RCT | Patients who were scheduled to have cardiac valve replacement surgery | 40 | Cefamandole 2g q6h | Vancomycin | PT3 d ≥ 32s | ─ | – |
| Yangco et al. (1987) [14] | England | RCT | Patients with suspected bacterial pneumonia | 92 | Latamoxef 2–4g q12h for mild renal impairment, 1-2g q12-24h for moderate to severe renal impairment | Ceftizoxime | Abnormality in clinical laboratory value (PT/PTT) | Adverse effects (hematemesis) | – |
| Huizinga (1988) [15] | South Africa | RCT | Severe intra-abdominal sepsis | 96 | Cefotetan 2g q12h | Ampicillin + gentamicin + metronidazole | PT > 5s | ─ | – |
| Williams et al. (1991) [16] | United States | RCT | Infections | 1109 | Cefotetan | Non-NMTT ABx e | PT > 5s above upper limit of normal range | Clinical bleeding episodes | – |
| Weitekamp et al. (1985) [17] | England | Prospective cohort | Healthy volunteers | 10 | Latamoxef 4g q24h, 2g q8h, 4g q8h | Cefotaxime | ─ | Clinical bleeding | 5 |
| Cohen et al. (1988) [18] | England | Prospective cohort | Patients with intra-abdominal sepsis who underwent surgery | 20 | Cefotetan | Cephadrin+ metronidazole or gentamicin + penicillin + metronidazole | INRf > 1.2 | ─ | 4 |
| Grasela et al. (1989) [19] | United States | Prospective cohort | Patients who require IV antibiotic therapy for intra-abdominal or obstetric-gynecologic process | 970 | Cefotetan | 1) Aminoglycoside + antianaerobic 2) cefoxitin | PT > 2s over baseline | Requiring transfusion | 6 |
| Goss et al. (1992) [20] | United States | Prospective cohort | Patients with cancer, fever, granulocytopenia, intraabdominal infection, nosocomial pneumonia | 460 | Cefotetan or cefoperazone | Non-NMTT ABx g | PT > 14s | Decrease in hemoglobin 10g/L or greater over a 24-hour period/required vitamin K/transfusion | 6 |
| Baxter et al. (1985) [21] | United States | Retrospective cohort | Abdominal sepsis | 94 | Moxalactam 2g q8h | Tobramycin + clindamycin | PT > 2s over baseline | Requiring transfusion of 1 to 2 units of packed red cells | 5 |
| Meyers et al. (1985) [22] | United States | Retrospective cohort | Infections | 4948 | Moxalactam or cefoperazone | Ceftazidime | Increased PT | Clinical bleeding | 4 |
| Bertino et al. (1986) [23] | United States | Retrospective cohort | ─ h | 41 | Cefamandole | Nafcillin/oxacillin | PT > 2s above the highest control end point | ─ | 7 |
| Brown et al. (1986) [24] | United States | Retrospective cohort | ─ | 755 for bleeding 1318 for increased PT | Cefamandole or moxalactam | Penicillin + cefoxitin | Increase in PT/PTT | Presence of grossly observable blood | 7 |
| Strom et al. (1999) [25] | England | Retrospective cohort | Infections | 853 | Cefoperazone | 1) Ceftizoxime/ Cefotaxime 2) Ceftazidime | PT + 2s, 5s, 15s PT×1.25, 1.5, 2 from baseline | Microscopically observed blood, grossly observed blood without transfusions, grossly observed blood with transfusions, cerebral hemorrhage, death from bleeding | 7 |
| Chen et al. (2016) [26] | Taiwan | Case-control | Patients hospitalized due to a hemorrhagic event subsequent to the use of antibiotics in ER | 6191 | Cefoperazone or cefmetazole | Non-NMTT ABx i | ─ | Diagnosis code of hemorrhage | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.H.; Kim, S.; Kim, M.S.; Yu, Y.M.; Kim, G.H.; Lee, J.S.; Lee, E. The Association Between Cephalosporin and Hypoprothrombinemia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3937. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16203937
Park GH, Kim S, Kim MS, Yu YM, Kim GH, Lee JS, Lee E. The Association Between Cephalosporin and Hypoprothrombinemia: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2019; 16(20):3937. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16203937
Chicago/Turabian StylePark, Gi Hyue, Seungyeon Kim, Min Soo Kim, Yun Mi Yu, Gun Hee Kim, Jeong Sang Lee, and Euni Lee. 2019. "The Association Between Cephalosporin and Hypoprothrombinemia: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 16, no. 20: 3937. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16203937





