Risk of Cardiovascular Mortality in Relation to Increased Total Serum IgE Levels in Older Adults: A Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Total Serum IgE Allergen
2.3. Identification of Death Cases
2.4. Variables of Interest
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pate, M.B.; Smith, J.K.; Chi, D.S.; Krishnaswamy, G. Regulation and dysregulation of immunoglobulin E: A molecular and clinical perspective. Clin. Mol. Allergy CMA 2010, 8, 3. [Google Scholar] [CrossRef]
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Ann. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Immunoglobulin E (IgE) and ischemic heart disease. Which came first, the chicken or the egg? Ann. Med. 2014, 46, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J.; Witztum, J.L. Is atherosclerosis an allergic disease? Circ. Res. 2011, 109, 1103–1104. [Google Scholar] [CrossRef] [Green Version]
- Bot, I.; Biessen, E.A. Mast cells in atherosclerosis. Thromb. Haemost. 2011, 106, 820–826. [Google Scholar] [PubMed] [Green Version]
- Wang, J.; Cheng, X.; Xiang, M.X.; Alanne-Kinnunen, M.; Wang, J.A.; Chen, H.; He, A.; Sun, X.; Lin, Y.; Tang, T.T.; et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe-/- mice. J. Clin. Investig. 2011, 121, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Jong, G.P.; Wang, Y.F.; Tsai, F.J.; Tsai, C.H.; Wu, C.L.; Liu, R.H.; Hsieh, D.J.; Ko, F.Y.; Huang, C.Y.; Lee, S.D. Immunoglobulin E and matrix metalloproteinase-9 in patients with different stages of coronary artery diseases. Chin. J. Physiol. 2007, 50, 277–282. [Google Scholar] [PubMed]
- Korkmaz, M.E.; Oto, A.; Saraclar, Y.; Oram, E.; Oram, A.; Ugurlu, S.; Karamehmetoglu, A.; Karaagaoglu, E. Levels of IgE in the serum of patients with coronary arterial disease. Int. J. Cardiol. 1991, 31, 199–204. [Google Scholar] [CrossRef]
- Tokac, M.; Ozdemir, A.; Yazici, M.; Altunkeser, B.B.; Duzenli, A.; Reisli, I.; Ozdemir, K. Is the beneficial effect of preinfarction angina related to an immune response? Jpn. Heart J. 2004, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, O.; Gul, C.; Altun, A.; Ozbay, G. Increased immunoglobulin E response in acute coronary syndromes. Angiology 2003, 54, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.D.; Criqui, M.H.; Feigelson, H.S.; McCann, T.J.; Hamburger, R.N. IgE predicts future nonfatal myocardial infarction in men. J. Clin. Epidemiol. 1996, 49, 203–209. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 5 May 2019).
- Shiue, I. Are higher serum IgE concentrations associated with adult cardiovascular disease? Int. J. Cardiol. 2013, 168, 1580–1581. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Lee, E.R.; Hamburger, R.N.; Klauber, M.R.; Coughlin, S.S. IgE and cardiovascular disease. Results from a population-based study. Am. J. Med. 1987, 82, 964–968. [Google Scholar] [CrossRef]
- Sinkiewicz, W.; Blazejewski, J.; Bujak, R.; Kubica, J.; Dudziak, J. Immunoglobulin E in patients with ischemic heart disease. Cardiol. J. 2008, 15, 122–128. [Google Scholar] [PubMed]
- Guo, X.; Yuan, S.; Liu, Y.; Zeng, Y.; Xie, H.; Liu, Z.; Zhang, S.; Fang, Q.; Wang, J.; Shen, Z. Serum IgE levels are associated with coronary artery disease severity. Atherosclerosis 2016, 251, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sinkiewicz, W.; Blazejewski, J.; Bujak, R.; Zekanowska, E.; Sobanski, P.; Kubica, J.; Dudziak, J.; Karasek, D.; Malyszka, P.; Balak, W.; et al. Immunoglobulin E as a marker of the atherothrombotic process in patients with acute myocardial infarction. Cardiol. J. 2007, 14, 266–273. [Google Scholar] [PubMed]
- Potaczek, D.P.; Kabesch, M. Current concepts of IgE regulation and impact of genetic determinants. Clin. Exp. Allergy J. Bri. Soc. Allergy Clin. Immunol. 2012, 42, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Martin, D.O.; Larson, M.G.; Levy, D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann. Intern. Med. 1998, 129, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
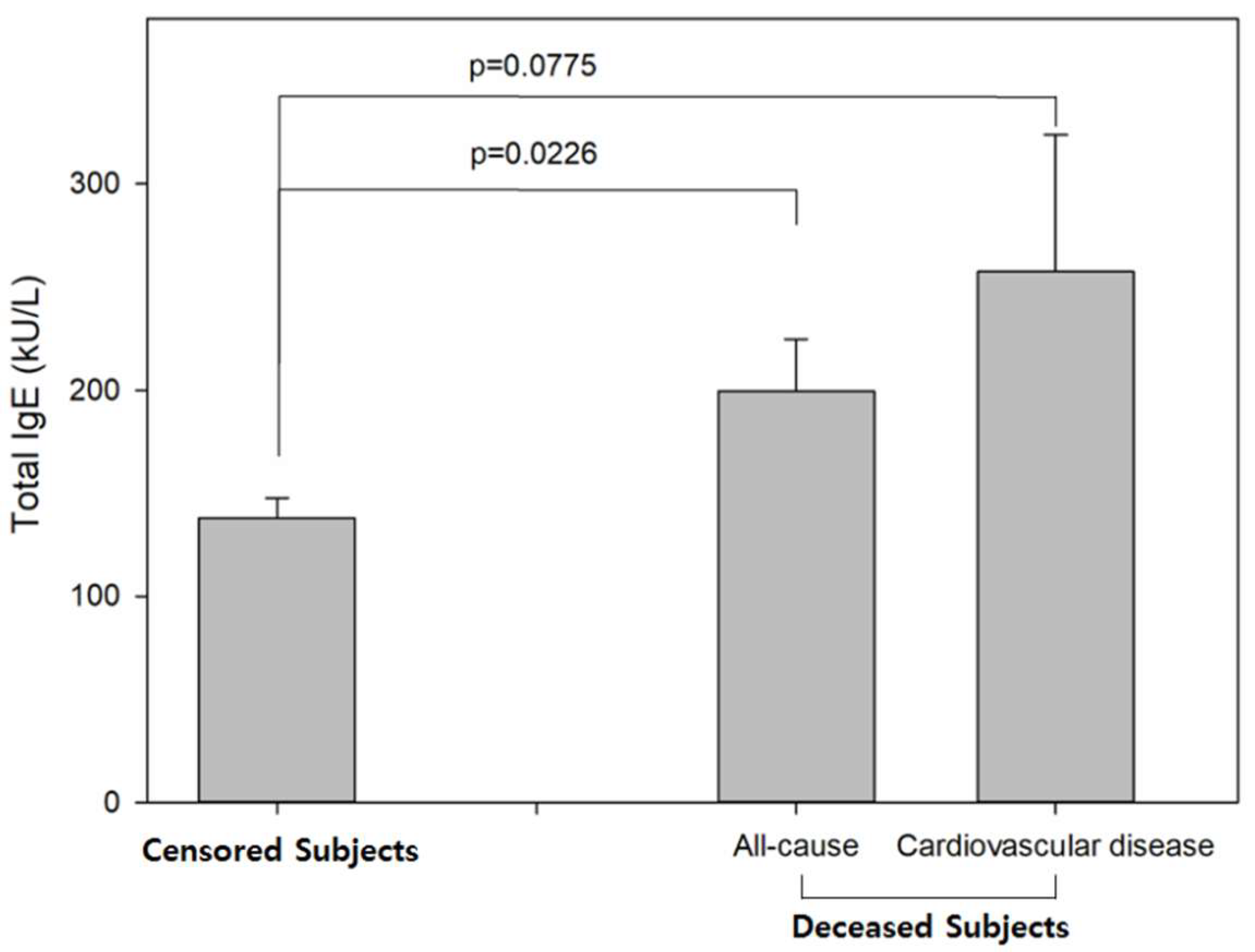
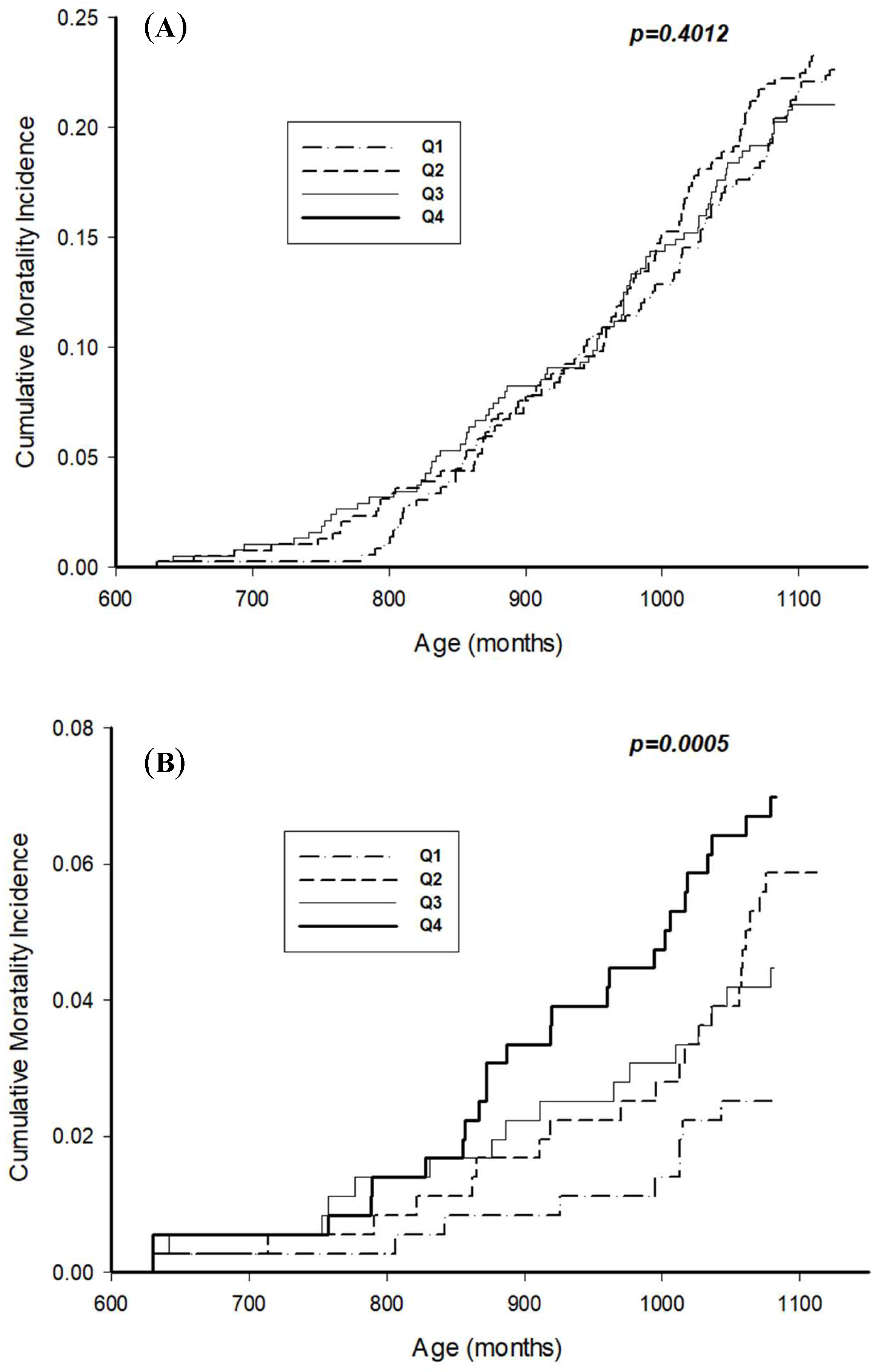
| No. of All Participants | Censored (n = 1065) No. (%) | Deceased (n = 431) No. (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Age (year) | ||||||
| 50–59 | 425 | 390 | (91.76) | 35 | (8.24) | <0.0001 |
| 60–69 | 472 | 383 | (81.14) | 89 | (18.86) | |
| 70–79 | 350 | 218 | (62.29) | 132 | (37.71) | |
| ≥80 | 249 | 74 | (29.72) | 175 | (70.28) | |
| Sex | ||||||
| Male | 752 | 517 | (68.75) | 235 | (31.25) | 0.0362 |
| Female | 744 | 548 | (73.66) | 196 | (26.34) | |
| Ethnicity | ||||||
| White | 847 | 555 | (65.53) | 292 | (34.47) | <0.0001 |
| Black | 338 | 255 | (75.44) | 83 | (24.56) | |
| Hispanic | 279 | 227 | (81.36) | 52 | (18.64) | |
| Others | 32 | 28 | (87.50) | 4 | (12.50) | |
| Family income ($) | ||||||
| <20,000 | 484 | 292 | (60.33) | 192 | (39.67) | <0.0001 |
| ≥20,000 | 1012 | 773 | (76.38) | 239 | (23.62) | |
| Educational attainment | ||||||
| High school or less | 565 | 369 | (65.31) | 196 | (34.69) | 0.0002 |
| High school graduate | 482 | 351 | (72.82) | 131 | (27.18) | |
| College or more | 449 | 345 | (76.84) | 104 | (23.16) | |
| Cigarette smoking | ||||||
| Current smoker | 303 | 217 | (71.62) | 86 | (28.38) | 0.0392 |
| Former smoker | 530 | 357 | (67.36) | 173 | (32.64) | |
| Never smoked | 663 | 491 | (74.06) | 172 | (25.94) | |
| Moderate physical activity | ||||||
| Yes | 664 | 515 | (77.56) | 149 | (22.44) | <0.0001 |
| No | 746 | 519 | (69.57) | 227 | (30.43) | |
| Unable | 86 | 31 | (36.05) | 55 | (63.95) | |
| BMI (kg/m2) | ||||||
| <18.5 | 18 | 9 | (50.00) | 9 | (50.00) | 0.0006 |
| 18.5–24.9 | 389 | 257 | (66.07) | 132 | (33.93) | |
| 25–29.9 | 517 | 361 | (69.83) | 156 | (30.17) | |
| ≥30.0 | 572 | 438 | (76.57) | 134 | (23.34) | |
| History of disease | ||||||
| Hypertension | ||||||
| Yes | 574 | 455 | (79.27) | 119 | (20.73) | <0.0001 |
| No | 922 | 610 | (66.16) | 312 | (33.84) | |
| Dyslipidemia | ||||||
| Yes | 802 | 581 | (72.44) | 221 | (27.56) | 0.2496 |
| No | 694 | 484 | (69.74) | 210 | (30.26) | |
| Diabetes mellitus | ||||||
| Yes | 330 | 215 | (65.15) | 115 | (34.85) | 0.0061 |
| No | 1166 | 850 | (72.90) | 316 | (27.10) | |
| Allergy history | ||||||
| Yes | 410 | 306 | (74.63) | 104 | (25.37) | 0.0707 |
| No | 1086 | 759 | (69.89) | 327 | (30.11) | |
| All-Cause Mortality | Cardiovascular Disease Mortality | |||||
|---|---|---|---|---|---|---|
| No. Events (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | No. Events (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Total IgE Quartile (kU/L) | ||||||
| Quartile 1 (≤16.80) | 105 (29.3) | 1 [Reference] | 1 [Reference] | 14 (3.9) | 1 [Reference] | 1 [Reference] |
| Quartile 2 (16.81–44.10) | 110 (28.42) | 0.81 (0.61–1.07) | 0.90 (0.71–1.14) | 23 (5.9) | 1.98 (0.90–4.36) | 2.56 (1.10–5.94) |
| Quartile 3 (44.11–123.00) | 101 (26.93) | 1.01 (0.63–1.61) | 1.04 (0.65–1.66) | 18 (4.8) | 1.24 (0.55–2.80) | 1.51 (0.69–3.31) |
| Quartile 4 (≥123.01) | 116 (30.45) | 1.14 (0.83–1.56) | 1.04 (0.64–1.69) | 30 (7.9) | 3.02 (1.77–5.13) | 3.19 (1.71–5.96) |
| All-Cause Mortality | Cardiovascular Disease Mortality | |||||
|---|---|---|---|---|---|---|
| No. Events (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | No. Events (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Subjects with Baseline Cardiovascular Disease | ||||||
| Total IgE Quartile (kU/L) | ||||||
| Quartile 1 (≤16.80) | 33 (47.14) | 1 (Reference) | 1 (Reference) | 7 (10.00) | 1 (Reference) | 1 (Reference) |
| Quartile 2 (16.81–44.10) | 26 (41.27) | 0.83 (0.37–1.82) | 1.37 (0.60–3.15) | 9 (14.29) | 2.14 (0.70–6.52) | 3.65 (0.87–15.33) |
| Quartile 3 (44.11–123.00) | 34 (49.28) | 1.01 (0.53–1.93) | 1.65 (0.86–2.50) | 10 (14.49) | 0.99 (0.42–2.30) | 2.16 (0.76–6.13) |
| Quartile 4 (≥123.01) | 39 (53.42) | 0.69 (0.38–1.28) | 1.13 (0.62–2.05) | 15 (20.55) | 1.37 (0.69–2.69) | 2.53 (0.88–7.24) |
| Subjects without Baseline Cardiovascular Disease | ||||||
| Total IgE Quartile (kU/L) | ||||||
| Quartile 1 (≤16.80) | 72 (25.00) | 1 (Reference) | 1 (Reference) | 7 (2.43) | 1 (Reference) | 1 (Reference) |
| Quartile 2 (16.81–44.10) | 84 (25.93) | 0.90 (0.61–1.35) | 0.92 (0.66–1.30) | 14 (4.32) | 2.88 (0.80–10.36) | 3.43 (1.08–10.94) |
| Quartile 3 (44.11–123.00) | 67 (21.90) | 1.00 (0.50–1.99) | 0.98 (0.52–1.84) | 8 (2.61) | 1.65 (0.36–7.64) | 1.95 (0.38–9.87) |
| Quartile 4 (≥123.01) | 77 (25.00) | 1.33 (0.91–1.95) | 1.06 (0.55–2.04) | 15 (4.87) | 5.57 (1.86–24.61) | 6.51 (1.81–23.43) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.-B.; Min, J.-Y. Risk of Cardiovascular Mortality in Relation to Increased Total Serum IgE Levels in Older Adults: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 4350. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16224350
Min K-B, Min J-Y. Risk of Cardiovascular Mortality in Relation to Increased Total Serum IgE Levels in Older Adults: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(22):4350. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16224350
Chicago/Turabian StyleMin, Kyoung-Bok, and Jin-Young Min. 2019. "Risk of Cardiovascular Mortality in Relation to Increased Total Serum IgE Levels in Older Adults: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 16, no. 22: 4350. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16224350




