3.1. Effects of Different Gelatin Concentrations, Bacterial Inoculation Rates and Bioindicator Concentrations on Bacteriocin Production
According to a previous study, low concentrations (less than 2%,
w/
v) of alginate used as the supporting gel or their mixture with other gels resulted in low viscosity and crosslinking sites did not create an uniform microencapsulated capsule. Whereas, the extrusion of alginate, or their mixture with other gels through a syringe at the high concentrations (3%,
w/
v), was difficult due to high viscosity [
3]. Hence, the present study selected 2.5% (
w/
v) sodium alginate as the optimum concentration of biopolymer matrices ALG-GEL.
L. plantarum SC01 was microencapsulated in ALG-GEL and tested for its antimicrobial activity. The effects of different gelatin concentrations (6%, 8%, 10%, 12%, and 14%
w/
v), different bacterial inoculation rates (1%, 2%, 3%, and 4%
w/
v), and different bioindicator concentrations (10
4, 10
5, 10
6, 10
7, and 10
8 CFU/mL) on Bacteriocin production of microencapsulated LAB were investigated. The effect of gelatin concentration mixed with 2.5% alginate on antimicrobial activity of Bacteriocin-containing supernatants are shown in
Table 1. In particular conditions, the formation of a mixed gel of alginate and gelatin is obtained.
There was a significant increase (
p < 0.05) in bio-indicator growth, compared to different gelatin concentrations under similar conditions. An increase in gelatin concentration mix, during microencapsulation, increased the growth of bio-indicators. This study clearly showed that
L. plantarum SC01 was microencapsulated in ALG-GEL (2.5% (
w/
v) alginate and 6.0% (
w/
v) gelatin), producing highly active compound that inhibited the growth of bio-indicator. The increasing of gelatin concentration will increase the viscosity of the solution and cause difficulties in the process of wrapping, thus greatly influences the quality of the package. This also greatly affects the fertility of antimicrobial compounds of bacteria [
25]. Al-muhanna et al. (2015) reported that, as the concentration of gelatin increases, its solubility in the liquid gelatin-alginate will be reduced, worsening the ability to link with alginate gel [
26]. Meanwhile, another study showed that the gelation time of ALG-GEL decreased with increasing gelatin content and buffer solution with Ca
2+ had a significant effect on the release of ALG-GEL capsules [
27].
Regarding the bacteria inoculation rate, it should be noted that the antagonistic compound produced by LAB was due to the bacterial inoculation rate [
28]. For instance, Silva et al. showed that three Bacteriocin-producing strains had high antimicrobial activity when inoculating the LAB at 1.0% (
v/
v) into MRS broth medium [
29]. In this study, four bacterial concentrations (1%, 2%, 3%, and 4%,
v/
v) were conducted in an attempt to identify a novel approach to optimize bacterial inoculation rate in producing antibacterial compounds.
L. plantarum SC01 was grown in MRSOPTSC01 broth at 37 °C for 48 h. CFS were microencapsulated in ALG-GEL (2.5% alginate and 6.0% gelatin,
w/
v), and the effect of
L. plantarum SC01 inoculation rate on Bacteriocin production are showed in
Table 2.
The highest percentages of inhibition were recorded at L. plantarum SC01 concentrations at 3% and 4% (v/v). To be specific, mean resistance to E. coli, S. aureus, B. subtilis respectively peaked at 56.85%, 56.79%, and 61.31%, corresponding to 3% L. plantarum SC01 inoculated. Against Salmonella, and L. monocytogenes, compared to other rates, 4% L. plantarum SC01 seems to inhibit the two bacteria the most, at mean rates of 63.27%, and 55.38%. However, except for results for S. aureus, differences between inhibitions produced by L. plantarum SC01 inoculated at the rate of 3% and 4% were not statistically significant, suggesting that increasing inoculation rate from 3% to 4% yielded diminished inhibitory effects. In an attempt to facilitate large-scale industrial production, inoculation rate of 3.0% was chosen to test the effect of factors on LAB antimicrobial activity.
Table 3 shows the growth of
E. coli,
Salmonella,
S. aureus,
B. subtilis, and
L. monocytogenes at an initial level of 10
4–10
8 CFU/mL inhibited by Bacteriocin produced by
L. plantarum SC01 microencapsulated in ALG-GEL.
For
Salmonella and
S. aureus (10
4 CFU/mL), it was shown that Bacteriocin allowed initial growth of the bio-indicators, with the low and medium concentration (10
4–10
7 CFU/mL), and inhibited the growth of the pathogens with the highest concentration of 10
8 CFU/mL. For the other bacteria, the inhibitor percentages are not significantly different at the initial level of 10
4–10
8 CFU/mL. This experiment showed that the inhibitory ability of antimicrobial compounds, produced by
L. plantarum SC01, was independent of the concentration of bio-indicator organisms. Meanwhile, previous studies have shown that the percentage of inhibition decreased when the concentration of indicator organisms increased [
30]. In this study, antimicrobial compounds produced by
L. plantarum SC01 is effective in inhibit pathogenic bacteria growth, whereas bio-indicator concentrations can be as dense as 10
8 CFU/mL.
3.2. Effect of Temperature, pH and Surfactants on Antimicrobial Activity
Different temperatures (37 °C, 60 °C, 80 °C, and 100 °C) were chosen to treat the CFS for 30 min and the percentage of antimicrobial activity are shown in
Table 4. The maximum percentage of inhibition reached approximately 60.0% after Bacteriocin-containing supernatants were treated at 80 °C and reached about 50.0% after being treated at 60 °C for 30 min. On the contrary, thermal treatment at 100 °C resulted in the loss of antimicrobial activity.
The result suggests that Bacteriocin-containing supernatants should be treated at temperature of 80 °C for the purpose of pathogen elimination and/or bacteria and bacterial spore removal. Similar results were recorded for a number of Bacteriocin, produced by
Lactobacillus spp. and lactocin NK24 produced by
Lc. lactis NK24, where the loss of its activity after 30 min at 100 °C achieved 87.5% [
31,
32]. In another study where lactocin MMFII was produced by
Lc. lactis MMFII, only 8.3% activity was recorded after 30 min at 110 °C and 25% after 30 min at 80 °C and 90 °C [
33]. Nisin, which is produced by
Lc. lactis subsp.
lactis WNC20, was incubated at 121 °C, pH 7.0 and then inactivated after 15 min whereas the sample that is incubated at pH 3.0 was not inactivated [
34]. Bozacin B14, produced by
Lc. lactis subsp.
lactis B14, was also inactivated after 10 min at 90 °C [
35].
Initial pH medium exerted a minor influence on production of
L. plantarum SC01 (
Table 5). Optimal levels of Bacteriocin were produced in MRSOPTSC01 at pH of 4.0. The percentage of antimicrobial activity reached 60.22% for
E. coli and reached around 50.0% for other bio-indicator. However, with the increase of pH from 2 to 10, the percentage of inhibition exhibited the same behavior. A similar study, Bacteriocins ST22Ch, ST153Ch and ST154CH, produced by three strains of
Lactobacillus sakei, isolated from salpicao, a fermented meat product from North-West of Portugal have a narrow spectrum of activity, are heat resistant and stable between pH 2.0 and pH 10.0 [
32].
Different surfactants were tested including Sodium dodecyl sulfate (SDS), Tween 20, Tween 80, Urea, and EDTA at final concentration of 1.0% (
w/
v) [
36,
37]. The highest percentage of inhibition was observed at EDTA while the lowest percentage of inhibition was observed at urea (
Table 6). In previous studies, Todorov et al. showed that activity of Bacteriocins was not affected by treatment with 1% Triton X-100, Tween 20, Tween 80, SDS, NaCl, urea and EDTA [
32]. The present study indicated that Bacteriocin could improve antimicrobial activity when treated with 1.0% (
w/
v) EDTA.
Several studies have demonstrated the diverse effects of LAB-loaded microencapsules, based on alginate gelatin, validating its potential use as a functional food. Microencapsulation of LAB and its antimicrobial potentiality had been observed by Li et al., Khandrae et al., Ariza et al., Corbo et al., Mei et al., and Léonard et al. [
17,
18,
38,
39,
40,
41]. Li et al. reported that encapsulation in alginate gelatin microcapsules successfully improved the survival of
L. casei ATCC 393 and could increase the cell numbers to 10
7 CFU/g in the dry state of microcapsules [
40]. In this way, Bacteriocin produced by
Lactobacillus plantarum microencapsulated in ALG-GEL capsules showed the potential as antagonistic compound.
3.3. Effect of Bacteriocin on Pork Meat Preservation
According to physical quality of pork meat during storage, after 12 h, the control sample started to emit odor and change in color, while the experiment samples treated with Bacteriocin-containing supernatants were odorless, elastic and had fresh, clean, and dry surfaces. Control samples at 24 h and 48 h could not maintain its original state, as well as impurities (
Figure 1). Meanwhile, experimental samples began to show signs of decomposition after 48 hours. This may be due to experimental meat samples are soaked in service only a single antibiotic should extend the survey period, the antibacterial activity also lost thereby not limiting the growth of bacteria present in the sample.
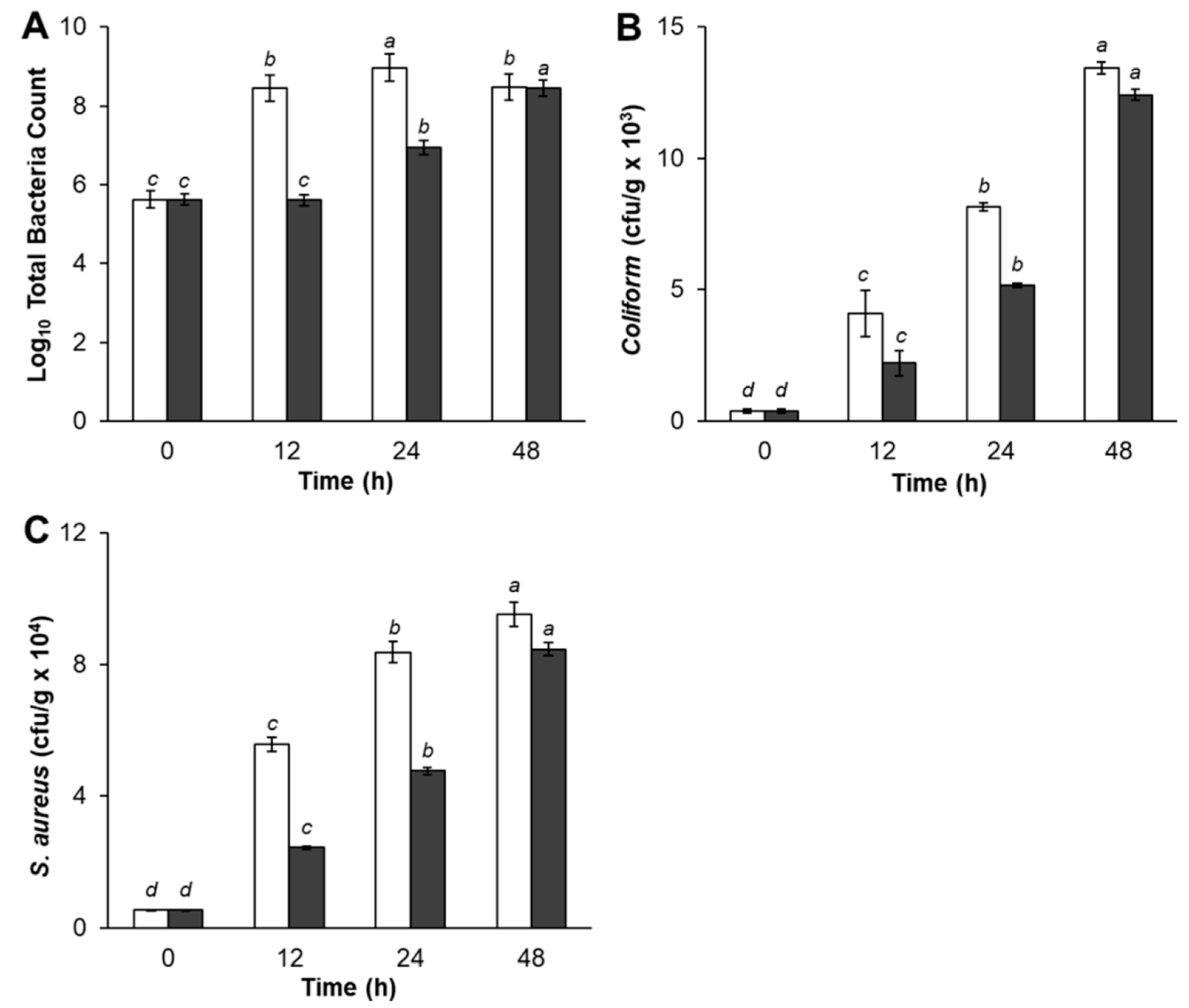
The microbial analyses of treated pork meat samples are shown in
Figure 2. As shown in
Figure 2A, TBC of control pork meat was significantly higher after storage for 12 h and 24 h, compared with experimental samples treated with Bacteriocin-containing supernatants. However, experimental samples TBC gradually increased by 24 h of storage period, then dramatically increased at the end of storage time. There was no significant difference between control and pork meat treatments in TBC at 48 h. Pork meat samples treated with Bacteriocin produced by
L. plantarum SC01 exhibited a slight increase in TBC (2.9 × 10
8, CFU/g) at 12 h compared with control (4.0 × 10
5, CFU/g). The inhibition of pathogenic bacteria growth was decreased after 24 h, and after 48 h, had the lowest rate of antimicrobial activity which TBC in control sample was 2.9 × 10
8 (CFU/g), while the samples were 2.7 × 10
8 (CFU/g), respectively. The same result was in agreement with Hamid et al. (2007), who reported that, during the storage of pork in nisin, TBC slightly increased during the first period of storage and then significantly increase till the end of storage period. That increase could be evidentially referred to the decrease in Bacteriocin production, which controls the rate of growth. This result indicated that adding Bacteriocin in pork meat had an inhibitory effect on pathogenic bacteria and the inhibition was apparent in fresh pork until it reached a maximum inhibition at 12 h of storage period.
Regarding the result of Total Coliform, data in
Figure 2B showed that Coliform bacteria was presented in both control and samples treated with Bacteriocin production. The control sample (4.1 × 10
3, CFU/g) had a significantly higher number of Coliform bacteria count at 12 h storage period as compared with the sample treatment (2.2 × 10
3, CFU/g). The Total Coliform increased after 24 h and 48 h. The quantity of Coliform bacteria in the samples increased, but lower than the control sample, indicating that antagonistic compound produced by
L. plantarum SC01 limited the growth of Coliform bacteria in samples. Our result was in accordance with Kim et al. (2004), who stated that fresh pork loins sprayed with organic acids, retarded pathogenic bacteria growth [
42]. This is obviously due to recontamination and suppressive effect of added starter cultures and probiotic
L. plantarum SC01 on Coliform bacteria.
Figure 2C showed that Bacteriocin-containing supernatants seem to prevent the growth of
S. aureus. During storage period,
S. aureus in treated sample gradually decreased after 12 h of storage period and insignificantly decreased at the end of the storage period compared with the control, in which the
S. aureus counts increased rapidly. At 12 h,
S. aureus in control sample was 5.6 × 10
4 (CFU/g). This figure in the treated sample was 2.4 × 10
4 (CFU/g) and increased after 24 h and 48 h. This result also showed that S.
aureus in samples were under control when treated with Bacteriocin. As predicted, antagonistic compound produced by
L. plantarum SC01 limited the growth of
S. aureus. Hereupon, the inhibitory effect upon
S. aureus organisms in treatment pork meat could be attributed to the attained high acidity of acidic metabolites end products, such as (lactic-acetic acids) and the Bacteriocin produced by
L. plantarum SC01. The results in this study indicated that samples were negative for
E.
coli and
Salmonella. At the same point of view, Dabiza et al. added the CFS of
L. rhamnosus showed the highest effect among
Lactobacilli against
E. coli,
S. aureus,
B. cereus, and
Aeromonas hydrophila [
43].
The result showing that antagonistic compound, produced by L. plantarum SC01, exhibited the highest capacity to preserve pork meat at 12 h. Because at this time, the antimicrobial compound remained active, inhibited the development of bacteria in the sample and limited the density of bacteria. By the time 24 h and 48 h, the bacteria began growth strongly, while the efficiency of antimicrobial compound diminished, creating favorable conditions for bacteria to develop.
The results from this study indicated that these antimicrobial compounds are only suitable for short-term storage. To be specific, the preservation time of pork meat should not exceed 2 days when stored in room temperature, which limits its potential application as bio-preservative. However, the results of this study will be a prerequisite for future developments of biological preservatives to improve the safety for users and increase usage time of food products.