Statistical Evaluation of Radiofrequency Exposure during Magnetic Resonant Imaging: Application of Whole-Body Individual Human Model and Body Motion in the Coil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Whole-Body Individual Modelling
2.2. Computational Method
2.2.1. Deterministic Simulations
2.2.2. Stochastic Dosimetry on Z-Axis Shift and Body Tilt
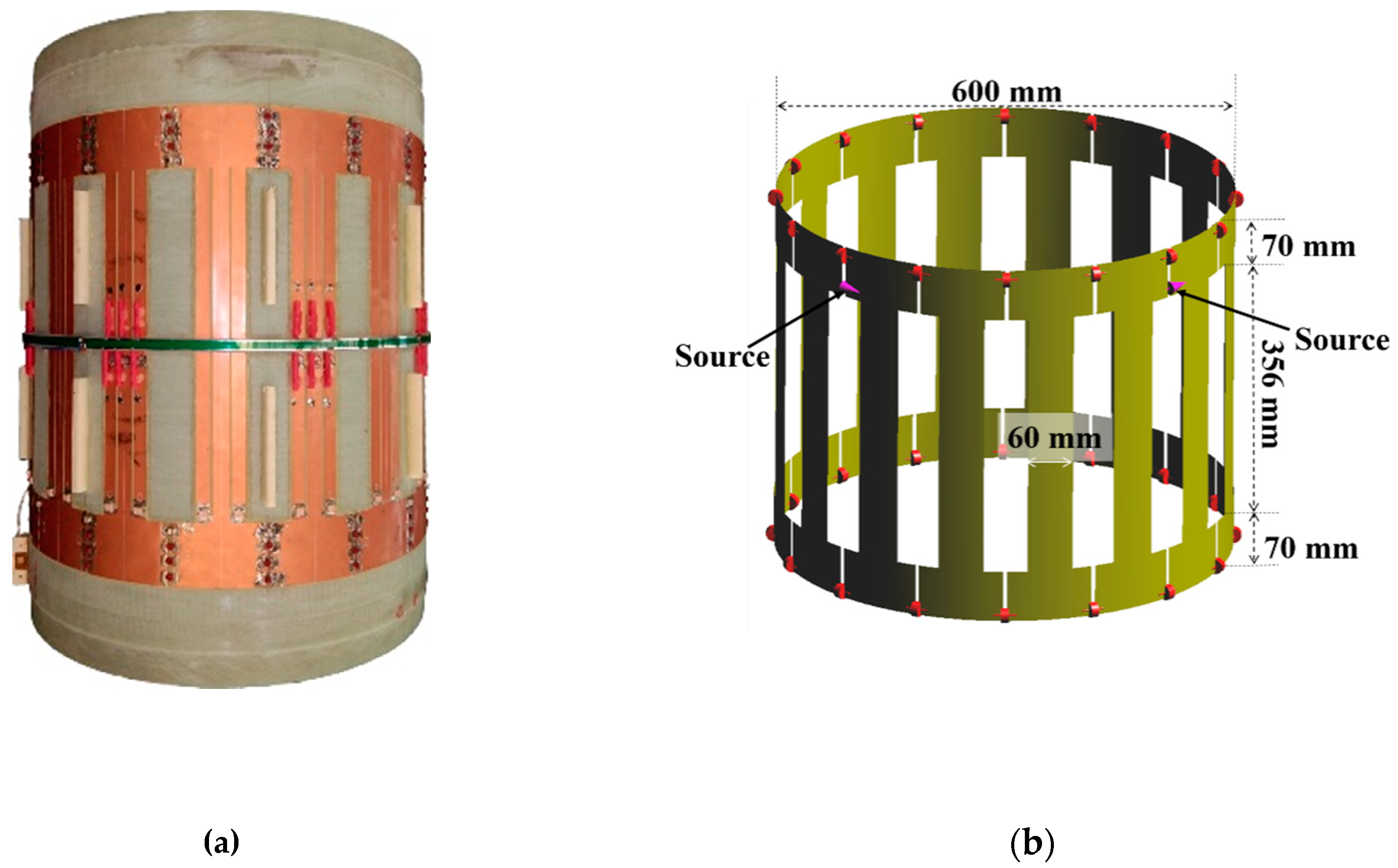
2.3. E-Field Measurement in the Empty Coil
3. Results
3.1. Whole-Body Individual Models
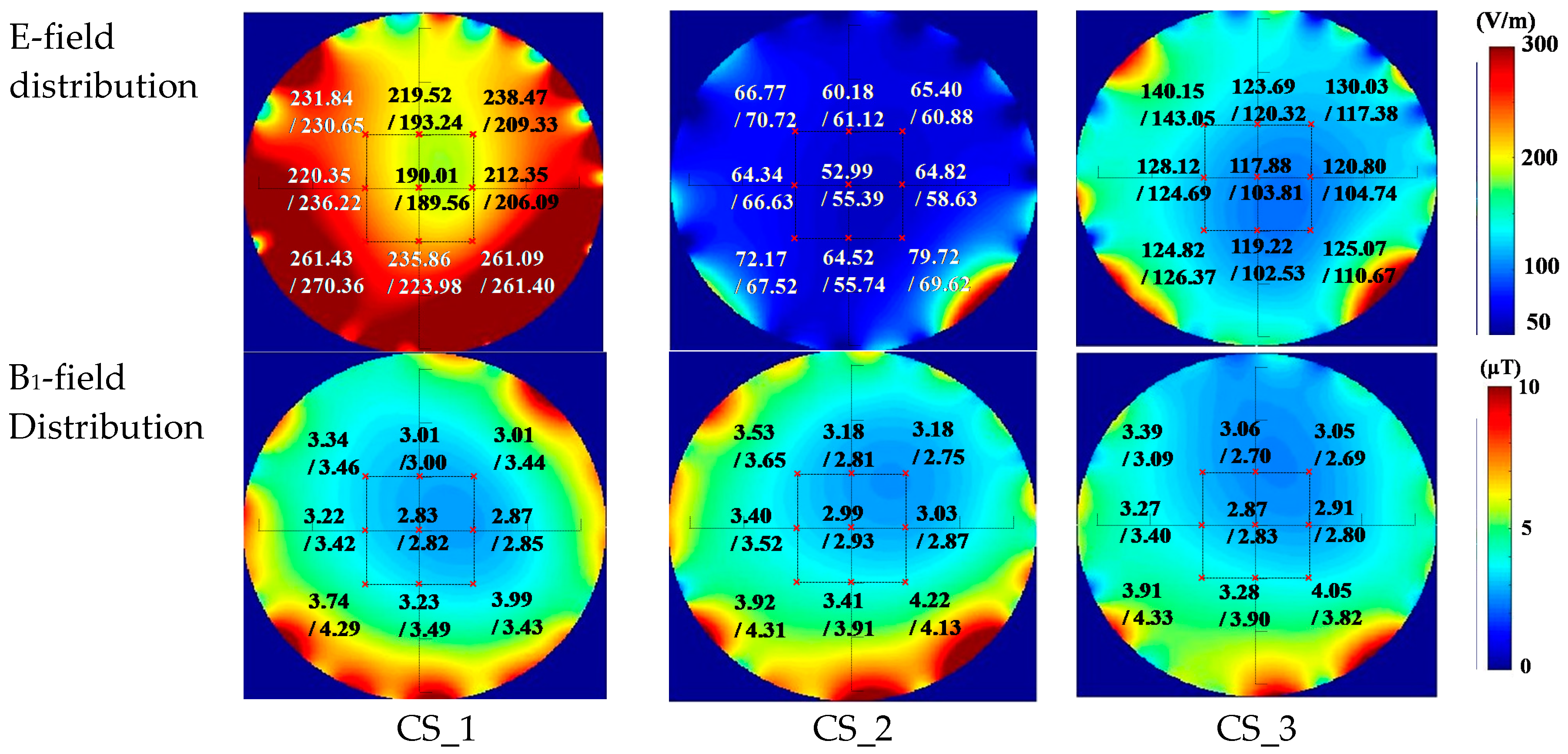
3.2. Comparison for the Simulation and the Measurement Results in the Empty Coil
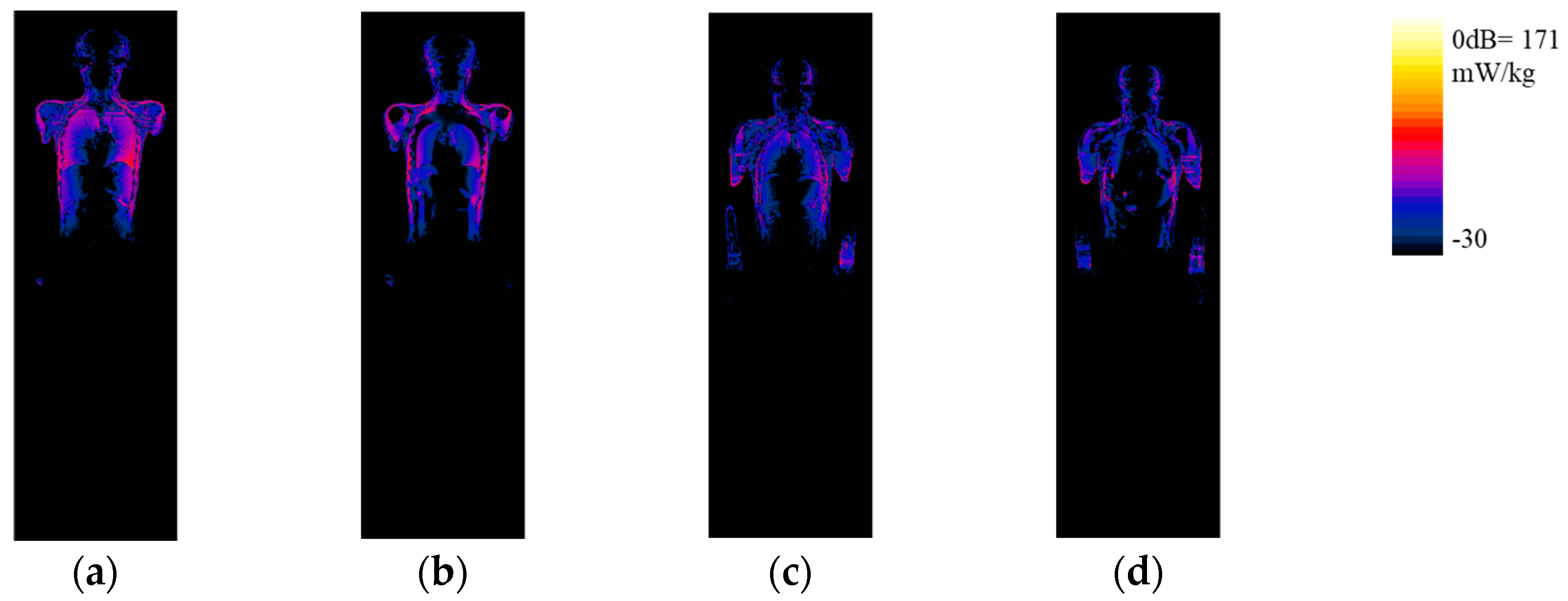
3.3. Deterministic Results
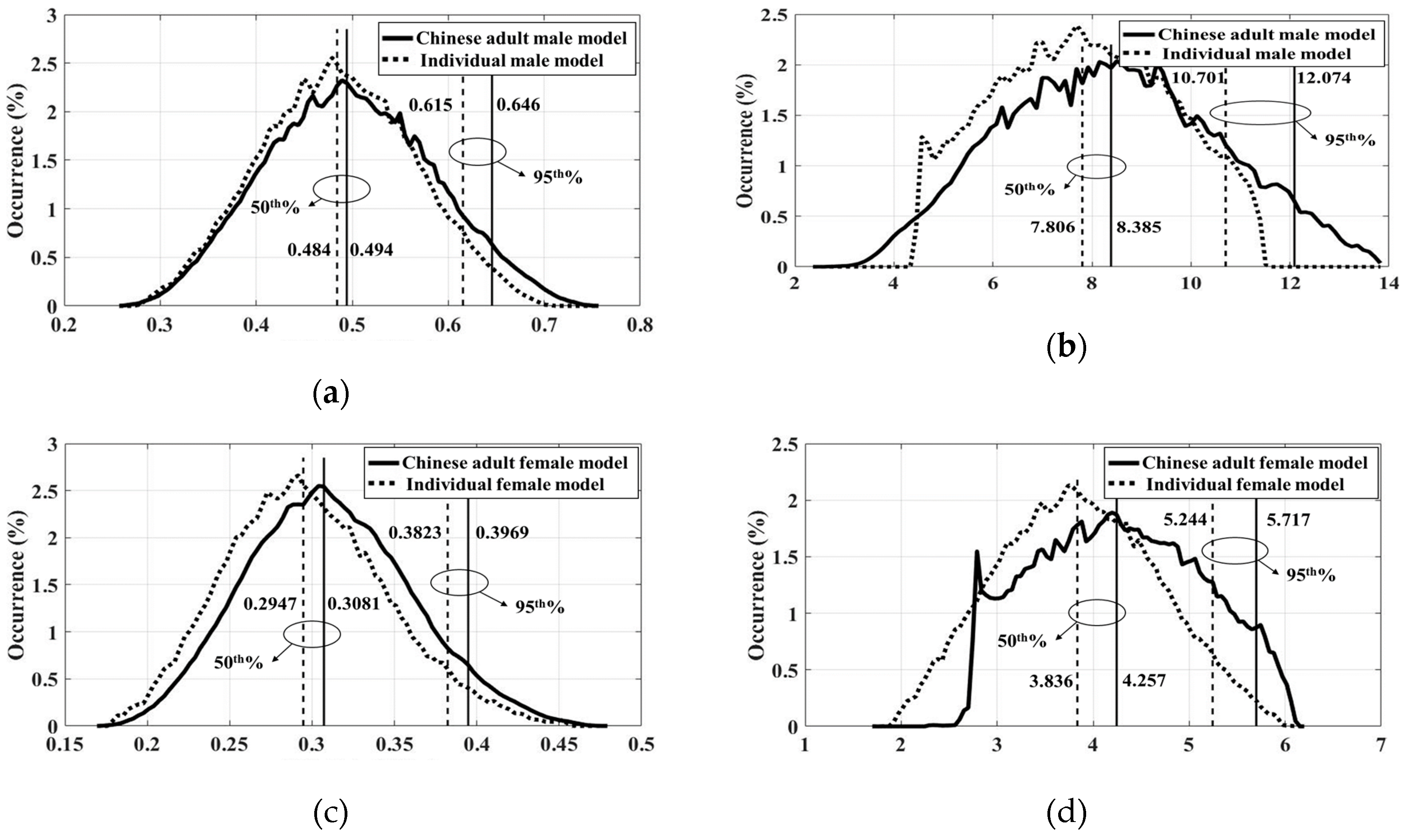
3.4. Statistical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McRobbie, D.W.; Moore, E.A.; Graves, M.J.; Prince, M.R. MRI from Picture to Proton; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Chen, X.; Steckner, M. Electromagnetic computation and modelling in MRI. Med. Phys. 2017, 44, 1186–1203. [Google Scholar] [CrossRef]
- Hand, J.W. Modelling the interaction of electromagnetic fields (10 MHz-10 GHz) with the human body: Methods and applications. Phys. Med. Biol. 2008, 53, R243–R286. [Google Scholar] [CrossRef] [PubMed]
- International Electrotechnical Commission. International standard, Medical Equipment IEC 60601-2-33: Particular Requirements for the Safety of Magnetic Resonance Equipment, 3rd ed.; IEC: Geneva, Switzerland, 2010. [Google Scholar]
- Wolf, S.; Diehl, D.; Gebhardt, M.; Mallow, J.; Speck, O. SAR simulations for high-field MRI: How much detail, effort, and accuracy is needed? Magn. Reson. Med. 2013, 69, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Liu, F.; Weber, E.; Crozier, S. Improving SAR estimations in MRI using subject-specific models. Phys. Med. Biol. 2012, 57, 8153–8171. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.C.; Neufeld, E.; Moser, H.; Huber, E.; Farcito, S.; Gerber, L.; Jedensjö, M.; Hilber, I.; Gennaro, F.D.; Lloyd, B.; et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: The Virtual Population 3.0. Phys. Med. Biol. 2014, 59, 5287–5303. [Google Scholar] [CrossRef]
- Li, X.; Rispoli, J.V. Toward 7T breast MRI clinical study: Safety assessment using simulation of heterogeneous breast models in RF exposure. Magn. Reson. Med. 2019, 81, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Homann, H.; Börnert, P.; Eggers, H.; Nehrke, K.; Dössel, O.; Graesslin, I. Toward individualized SAR models and in vivo validation. Magn. Reson. Med. 2011, 66, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shao, Q.; Yang, L. Simplified segmented human models for whole body and localised SAR evaluation of 20 MHz to 6 GHz electromagnetic field exposures. Radiat. Prot. Dosim. 2012, 153, 266–272. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Yang, L.; Lv, B.; Liu, J.; Varsier, N.; Hadjem, A. Generation of infant anatomical models for evaluating electromagnetic field exposures. Bioelectromagnetics 2015, 36, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zeng, P.; Wang, S. Statistical simulation of SAR variability with geometric and tissue property changes by using the unscented transform. Magn. Reson. Med. 2015, 73, 2357–2362. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.B.; Wu, T.N.; Li, C.S.; Chen, Z.H.; Liao, M.Y.; Li, M.C.; Yan, W.; Huang, H.; Yang, J.; et al. Deformable torso phantoms of Chinese adults for personalized anatomy modelling. J. Anat. 2018, 233, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Qiu, T.S.; Huo, L.; Yu, L.J.; Shi, H.C.; Zhang, Y.J.; Wang, H.K. Deformable Head Atlas of Chinese Adults Incorporating Inter-Subject Anatomical Variations. IEEE Access 2018, 6, 51392–51400. [Google Scholar] [CrossRef]
- Murbach, M.; Cabot, E.; Neufeld, E.; Gosselin, M.C.; Christ, A.; Kuster, N. Local SAR enhancements in anatomically correct children and adult models as a function of position within 1.5 T MR body coil. Prog. Biophys. Mol. Biol. 2011, 107, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Murbach, M.; Neufeld, E.; Kainz, W.; Pruessmann, K.P.; Kuster, N. Whole-body and local RF absorption in human models as a function of anatomy and position within 1.5 T MR body coil. Magn. Reson. Med. 2014, 71, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.; Scorretti, R.; Sabariego, R.V.; Geuzaine, C. Stochastic uncertainty quantification of the eddy current in human body by using polynomial chaos decomposition. IEEE Trans. Magn. 2012, 48, 451–454. [Google Scholar]
- Liorni, I.; Parazzini, M.; Fiocchi, S.; Ravazzani, P. Study of the influence of the orientation of a 50-Hz magnetic field on Fetal exposure using polynomial chaos decomposition. Int. J. Environ. Res. Public Health 2015, 12, 5934–5953. [Google Scholar] [CrossRef] [PubMed]
- Liorni, I.; Parazzini, M.; Varsier, N.; Hadjem, A.; Ravazzani, P.; Wiart, J. Exposure assessment of one-year-old child to 3G tablet in uplink mode and to 3G femtocell in downlink mode using polynomial chaos decomposition. Phys. Med. Biol. 2016, 61, 3237–3257. [Google Scholar] [CrossRef] [PubMed]
- Ghanmi, A.; Varsier, N.; Hadjem, A.; Conil, E.; Picon, O.; Wiart, J. Analysis of the influence of handset phone position on RF exposure of brain tissue. Bioelectromagnetics 2014, 35, 568–579. [Google Scholar] [CrossRef]
- Kersaudy, P.; Sudret, B.; Varsier, N.; Picon, O.; Wiart, J. A new surrogate modelling technique combining Kriging and polynomial chaos expansions–Application to uncertainty analysis in computational dosimetry. J. Comput. Phys. 2015, 286, 103–117. [Google Scholar] [CrossRef]
- Wiener, N. The homogeneous chaos. Am. J. Math. 1938, 60, 897–936. [Google Scholar] [CrossRef]
- Heimann, T.; Meinzer, H.P. Statistical shape models for 3D medical image segmentation: A review. Med. Image Anal. 2009, 13, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Turbosquid. Male and Female Anatomy Complete Pack (Textured). Available online: https://www.turbosquid.com/3d-models/male-female-anatomy-body-3d-max/602826/ (accessed on 4 April 2018).
- Cootes, T.F.; Taylor, C.J.; Cooper, D.H.; Graham, J. Active shape models-their training and application. Comput. Vis. Image Underst. 1995, 61, 38–59. [Google Scholar] [CrossRef]
- Wu, T.; Tan, L.; Shao, Q.; Li, Y.; Yang, L.; Zhao, C.; Xie, Y.; Zhang, S. Slice-based supine to standing postured deformation for Chinese anatomical models and the dosimetric results by wide band frequency electromagnetic field exposure: Morphing. Radiat. Prot. Dosim. 2012, 154, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.N.; Tan, L.W.; Shao, Q.; Zhang, C.; Zhao, C.; Li, Y.; Conil, E.; Hadjem, A.; Wiart, J.; Lu, B. Chinese adult anatomical models and the application in evaluation of RF exposures. Phys. Med. Biol. 2011, 56, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Hagness, S.C. Computational Electrodynamics: The Finite-Difference Time-Domain Method; Artech House: Boston, MA, USA; London, UK, 2005. [Google Scholar]
- Potter, M.; Bérenger, J.P. A Review of the Total Field/Scattered Field Technique for the FDTD Method. FERMAT 2017, 19, 1–13. [Google Scholar]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.A.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues, version 4.0; IT’IS Foundation: Zurich, Switzerland, 2018. [Google Scholar] [CrossRef]
- Wiart, J. Radio-Frequency Human Exposure Assessment: From Deterministic to Stochastic Methods; Wiley-IEEE Press: New York, NY, USA, 2016; pp. 119–155. [Google Scholar]
- Xiu, D.; Karniadakis, G.E. The Wiener—Askey polynomial chaos for stochastic differential equations. SIAM J. Sci. Comput. 2002, 24, 619–644. [Google Scholar] [CrossRef]
- Efron, B.; Hastie, T.; Johnstone, I.; Tibshirani, R. Least angle regression. Ann. Stat. 2004, 32, 407–499. [Google Scholar]
- Stone, M. Cross-validatory choice and assessment of statistical predictions. J. R. Stat. Soc. Ser. B Methodol. 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Blatman, G.; Sudret, B. Adaptive sparse polynomial chaos expansion based on least angle regression. J. Comput. Phys. 2011, 230, 2345–2367. [Google Scholar] [CrossRef]
- Iman, R.L.; Davenport, J.M.; Zeigler, D.K. Latin Hypercube Sampling (Program User’s Guide); [LHC, in FORTRAN]; Sandia Labs.: Albuquerque, NM, USA, 1980. [Google Scholar]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family-developmentof surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 2009, 55, N23–N38. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G. MRI safety and neuromodulation systems. In Neuromodulation; Academic Press: New York, NY, USA, 2009; pp. 243–281. [Google Scholar]
- Verleysen, M.; François, D. The curse of dimensionality in data mining and time series prediction. In International Work-Conference on Artificial Neural Networks; Springer: Berlin/Heidelberg, Germany, 2005; pp. 758–770. [Google Scholar]
- Capstick, M.; McRobbie, D.; Hand, J.; Christ, A.; Kühn, S.; Mild, K.H.; Cabot, E.; Li, Y.; Melzer, A.; Papadaki, A.; et al. An Investigation into Occupational Exposure to Electromagnetic Fields for Personnel Working with and Around Medical Magnetic Resonance Imaging Equipment. Report on Project VT/2007/017 of the European Commission Employment. 2008. Available online: http://www.itis.ethz.ch/downloads/VT2007017FinalReportv04.pdf (accessed on 25 March 2019).
- Li, B.K.; Liu, F.; Weber, E.; Crozier, S. Hybrid numerical techniques for the modelling of radio frequency coils in MRI. NMR Biomed. Int. J. Dev. Appl. Magn. Reson. In Vivo 2009, 22, 937–951. [Google Scholar]
- Kozlov, M.; Turner, R. A comparison of Ansoft HFSS and CST microwave studio simulation software for multi-channel coil design and SAR estimation at 7T MRI. Piers Online 2010, 6, 395–399. [Google Scholar] [CrossRef]
- Bottauscio, O.; Chiampi, M.; Hand, J.; Zilberti, L. A GPU computational code for eddy-current problems in voxel-based anatomy. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
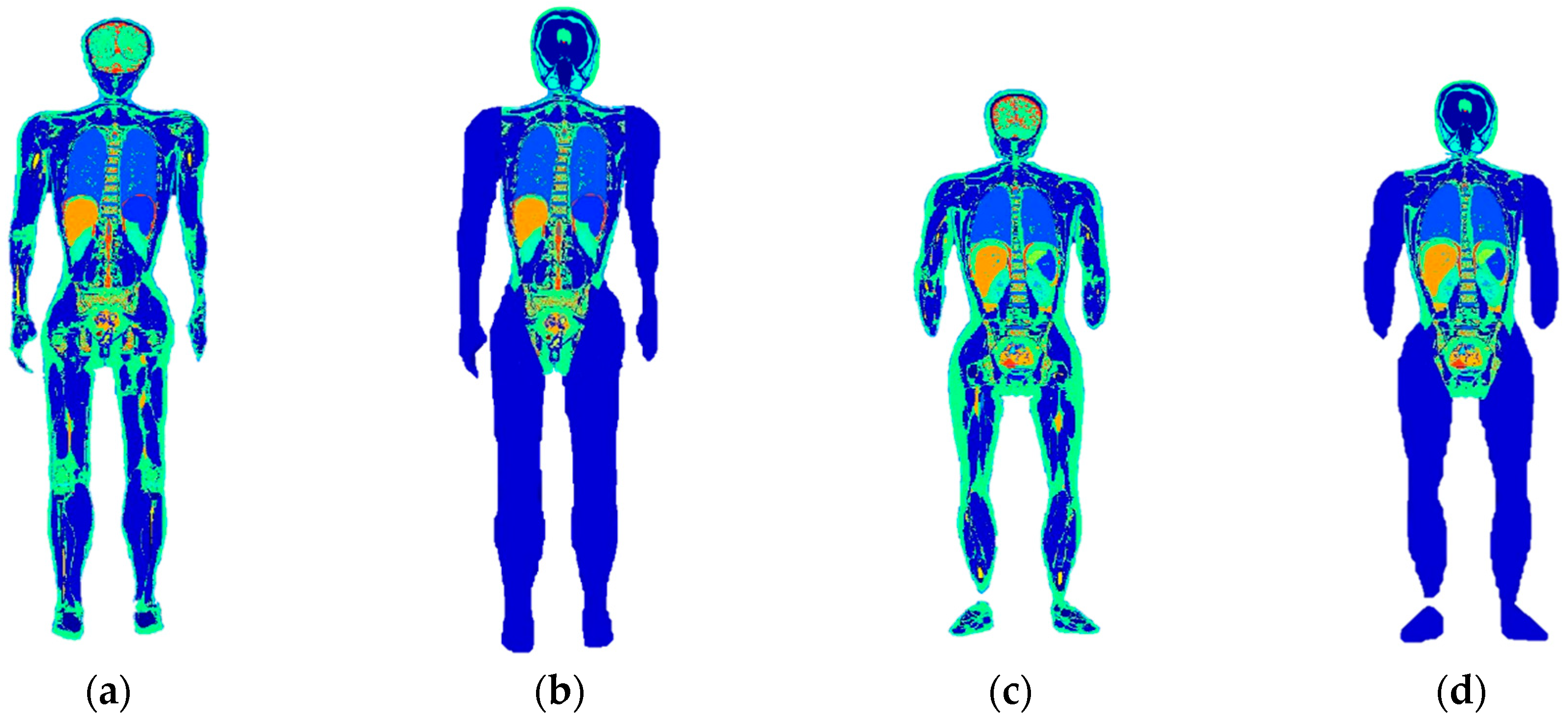



| Chinese Adult Male Model | Individualized Adult Male Model | Chinese Adult Female Model | Individualized Adult Female Model |
|---|---|---|---|
| Aqueous humor | Eyes | Aqueous humor | Eyes |
| Cornea | Cornea | ||
| Cortex_of_lens | Cortex_of_lens | ||
| Sclera | Sclera | ||
| Retina | Retina | ||
| Vitreous_body | Vitreous_body | ||
| Lens_nucleus | Lens_nucleus | ||
| Iris | Iris | ||
| Lacrimal_apparatus | Lacrimal_apparatus | ||
| Brain_stem | Brain | Brain_stem | Brain |
| Cerebral_dura_mater | Cerebral_dura_mater | ||
| Cerebral_grey_matter | Cerebral_grey_matter | ||
| Cerebral_white_matter | Cerebral_white_matter | ||
| Hippocampus | Hippocampus | ||
| Hypophysis | Hypophysis | ||
| Hypothalamus | Hypothalamus | ||
| Cerebellum | Cerebellum | ||
| Cerebrospinal_fluid | Cerebrospinal_fluid | ||
| Cartilage | Cartilage | Cartilage | Cartilage |
| Large_artery_wall | Large_artery_wall | ||
| Large_vein_wall | Large_vein_wall | ||
| Laryngeal_cartilages | Laryngeal_cartilages | ||
| Nerve | Nerve | ||
| Vestibulocochlear_nerve | Vestibulocochlear_nerve | ||
| Diploe | Skull | Diploe | Skull |
| Teeth | Teeth | ||
| Bile | — | Bile | — |
| Bladder | Bladder | Bladder | Bladder |
| Blood | Blood | Blood | Blood |
| Cholecyst | — | Cholecyst | — |
| Cortical_bone | Cortical_bone | Cortical_bone | Cortical_bone |
| Fat | Fat | Fat | Fat |
| Heart | Heart | Heart | Heart |
| Internal_ear | Internal_ear | Internal_ear | Internal_ear |
| Intervertebral_disc | Intervertebral_disc | Intervertebral_disc | Intervertebral_disc |
| Kidney | Kidney | Kidney | Kidney |
| Large_intestine | Intestines | Large_intestine | Intestines |
| Enteric_cavity | Enteric_cavity | ||
| Ligament | Ligament | Ligament | Ligament |
| Liver | Liver | Liver | Liver |
| Lung | Lung | Lung | Lung |
| Lymph_node | Lymph_node | Lymph_node | Lymph_node |
| — | — | Mammary_gland | Mammary_gland |
| Intrinsic_laryngeal_muscle | Muscle | Intrinsic_laryngeal_muscle | Muscle |
| Muscle_belly | Muscle_belly | ||
| Tongue | Tongue | ||
| Muscle_tendon | Muscle_tendon | ||
| Nucleus | Nucleus | Nucleus | Nucleus |
| Optical_nerve | Optical_nerve | Optical_nerve | Optical_nerve |
| Pancreas | Pancreas | Pancreas | Pancreas |
| Pineal_gland | Pineal_gland | Pineal_gland | Pineal_gland |
| Prostate | Prostate | — | — |
| Red_bone_marrow | Red_bone_marrow | Red_bone_marrow | Red_bone_marrow |
| Salivary_gland | Salivary_gland | Salivary_gland | Salivary_gland |
| Skin | Skin | Skin | Skin |
| Spinal_cord | Spinal_cord | Spinal_cord | Spinal_cord |
| Spinal_dura_mater | Spinal_dura_mater | Spinal_dura_mater | Spinal_dura_mater |
| Spleen | Spleen | Spleen | Spleen |
| Spongy_bone | Spongy_bone | Spongy_bone | Spongy_bone |
| Stomach | Stomach | Stomach | Stomach |
| Stomach lumen | Stomach lumen | ||
| Testis | Testis | — | — |
| Thoracic_gland | Thoracic_gland | — | — |
| Thyroid | Thyroid | Thyroid | Thyroid |
| Trachea | Trachea | Trachea | Trachea |
| Ureter | Ureter | Ureter | Ureter |
| Vessel | Vessel | — | — |
| Homogenized Tissues | Conductivity (S/m) | Relative Permittivity |
|---|---|---|
| Eyes | 1.24 | 70.96 |
| Brain | 0.69 | 72.35 |
| Cartilage | 0.60 | 66.76 |
| skull | 0.13 | 26.38 |
| Muscle | 0.69 | 72.24 |
| Stomach | 0.88 | 85.81 |
| Tissues | Chinese Adult Male Model (kg) | Individual Male Model (kg) | Weight Deviation (%) | Dice 1 (%) | Chinese Adult Female Model (kg) | Individual Female Model (kg) | Weight Deviation (%) | Dice (%) |
|---|---|---|---|---|---|---|---|---|
| Total weight | 63.26 | 66.70 | 5.44 | / | 53.47 | 54.99 | 2.84 | / |
| Fat | 21.54 | 23.32 | 8.26 | / | 17.10 | 18.81 | 10.00 | / |
| Muscle | 22.37 | 24.23 | 8.31 | / | 16.23 | 16.03 | −1.20 | / |
| Skin | 3.79 | 3.77 | −0.57 | / | 3.14 | 3.15 | 0.27 | / |
| Bones | 8.42 | 9.01 | 7.00 | / | 5.96 | 6.22 | 4.36 | / |
| Brain | 1.38 | 1.39 | −1.49 | 81.08 | 1.30 | 1.26 | −2.67 | 78.75 |
| Heart | 0.42 | 0.35 | −17.00 | 69.49 | 0.27 | 0.30 | 11.11 | 69.98 |
| Kidney | 0.26 | 0.29 | 11.53 | 69.55 | 0.22 | 0.19 | −13.63 | 63.83 |
| Liver | 2.05 | 1.84 | −10.24 | 63.17 | 1.17 | 1.02 | −12.80 | 61.76 |
| Lung | 1.02 | 0.90 | −11.76 | 63.39 | 0.93 | 0.86 | −7.76 | 68.87 |
| Spleen | 0.19 | 0.16 | −15.79 | 61.18 | 0.18 | 0.14 | −16.67 | 63.22 |
| Stomach | 0.76 | 0.74 | −2.63 | 72.32 | 0.59 | 0.67 | 13.55 | 69.70 |
| Human Models | wbSAR 1 | Deviation (%) 2 | hdSAR 3 | Deviation (%) | pSAR10g | Deviation (%) | Location of pSAR10g |
|---|---|---|---|---|---|---|---|
| Manually segmented male model | 0.48 mW/kg | −2.62 | 0.27 mW/kg | 3.20 | 8.39 mW/kg | −7.55 | (86, 85, 489) |
| Individual male model | 0.47 mW/kg | 0.28 mW/kg | 7.76 mW/kg | (101, 86, 492) | |||
| Manually segmented female model | 0.31 mW/kg | −3.06 | 0.21 mW/kg | 4.53 | 4.24 mW/kg | −9.09 | (93, 79, 461) |
| Individual female model | 0.30 mW/kg | 0.22 mW/kg | 3.85 mW/kg | (96, 82, 463) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, H.; Zhang, P.; Li, C.; Sun, J.; Chen, Z.; Xing, S.; Liang, P.; Wu, T. Statistical Evaluation of Radiofrequency Exposure during Magnetic Resonant Imaging: Application of Whole-Body Individual Human Model and Body Motion in the Coil. Int. J. Environ. Res. Public Health 2019, 16, 1069. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16061069
Liu W, Wang H, Zhang P, Li C, Sun J, Chen Z, Xing S, Liang P, Wu T. Statistical Evaluation of Radiofrequency Exposure during Magnetic Resonant Imaging: Application of Whole-Body Individual Human Model and Body Motion in the Coil. International Journal of Environmental Research and Public Health. 2019; 16(6):1069. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16061069
Chicago/Turabian StyleLiu, Wenli, Hongkai Wang, Pu Zhang, Chengwei Li, Jie Sun, Zhaofeng Chen, Shengkui Xing, Ping Liang, and Tongning Wu. 2019. "Statistical Evaluation of Radiofrequency Exposure during Magnetic Resonant Imaging: Application of Whole-Body Individual Human Model and Body Motion in the Coil" International Journal of Environmental Research and Public Health 16, no. 6: 1069. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16061069





