Recent Status and Methodological Quality of Return-to-Work Rates of Cancer Patients Reported in Japan: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Return-to-Work Rates
2.3. Methodological Quality Assessment
3. Results
3.1. Literature Search
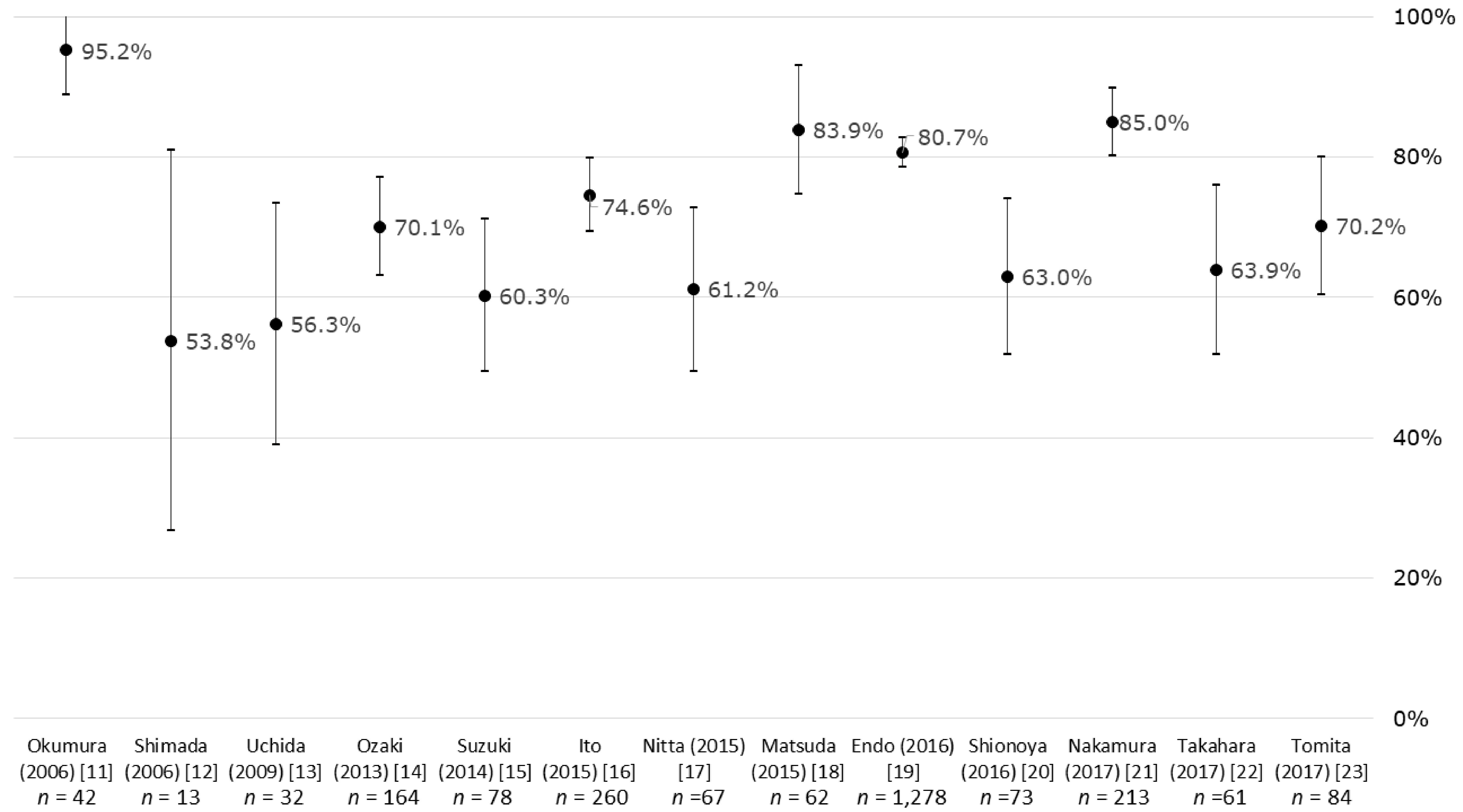
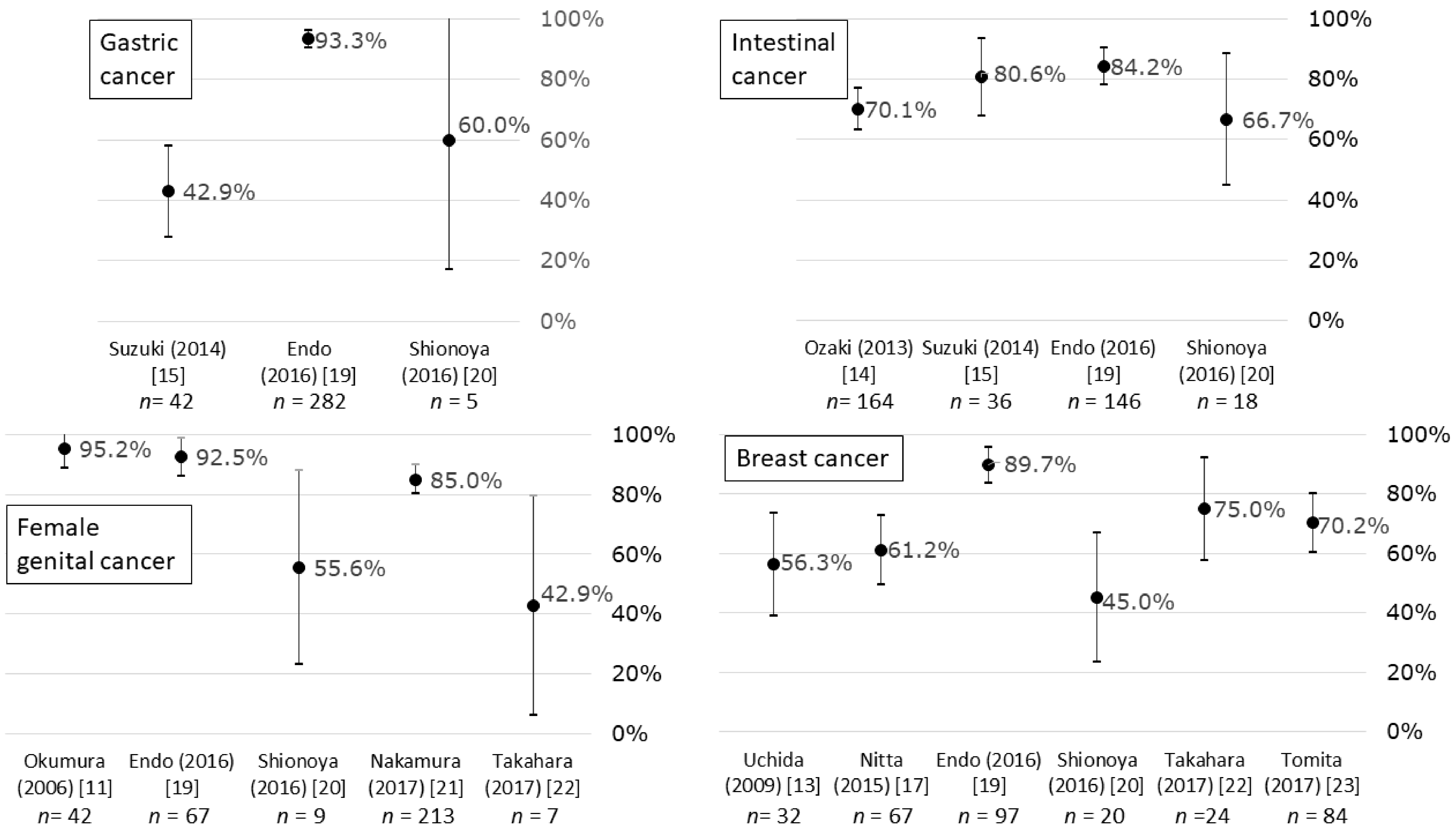
3.2. Return-to-Work Rates
3.3. Methodological Quality Assessment
3.3.1. RoBANS Domain 1 “Selection of Participants (Selection Bias)”
3.3.2. RoBANS Domain 2 “Confounding Variables (Selection Bias)”
3.3.3. RoBANS Domain 3 “Measurement of Exposure (Performance Bias)”
3.3.4. RoBANS Domain 4 “Blinding of Outcome Measurement (Detection Bias)”
3.3.5. RoBANS Domain 5 “Incomplete Outcome Data (Attrition Bias)”
3.3.6. RoBANS Domain 6 “Selective Outcome Reporting (Reporting Bias)”
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foundation for Promotion of Cancer Research Japan (FPCRJ). Cancer Statistics in Japan–2017; FPCRJ: Tokyo, Japan, 2018; Available online: https://ganjoho.jp/data/reg_stat/statistics/brochure/2017/cancer_statistics_2017.pdf (accessed on 7 February 2019).
- Center for Cancer Control and Information Services (CCCIS), National Cancer Center Japan (NCCJ). Monitoring Cancer Incidence in Japan, MCIJ 2013; CCCIS: Tokyo, Japan, 2016; Available online: https://ganjoho.jp/data/reg_stat/statistics/brochure/mcij2013_report_201806.pdf (accessed on 7 February 2019). (In Japanese)
- CCCIS, NCCJ. Monitoring of Cancer Incidence in Japan–Survival; CCCIS: Tokyo, Japan, 2016; Available online: https://ganjoho.jp/data/reg_stat/statistics/brochure/mcij2006-2008_report.pdf (accessed on 7 February 2019). (In Japanese)
- de Boer, A.G.; Taskila, T.; Ojajärvi, A.; van Dijk, F.J.; Verbeek, J.H. Cancer survivors and unemployment: A meta-analysis and meta-regression. JAMA 2009, 301, 753–762. [Google Scholar] [CrossRef]
- Mehnert, A. Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 2011, 77, 109–130. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Fugazzaro, S.; Bertozzi, L.; Bassi, M.C.; Pellegrini, M.; Vicentini, M.; Mazzini, E.; Costi, S. Return to work in European Cancer survivors: A systematic review. Support. Care Cancer 2018, 26, 2983–2994. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- van Muijen, P.; Weevers, N.L.; Snels, I.A.; Duijts, S.F.; Bruinvels, D.J.; Schellart, A.J.; van der Beek, A.J. Predictors of return to work and employment in cancer survivors: A systematic review. Eur. J. Cancer Care Engl. 2013, 22, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.S.; Ganz, P.A.; Pavlish, C.; Robbins, W.A. Young adult cancer survivors and work: A systematic review. J. Cancer Surviv. 2017, 11, 765–781. [Google Scholar] [CrossRef]
- Okumura, F.; Ogino, A.; Sasada, Y.; Nakata, N. Fujinka Gan Kanja no Syujutu-Go No Nichijo Seikatsu Jittai Chosa (Examination on Post-Operative Daily Life of Patients with Gynecological Cancer); Nihon Kango Gakkai Ronbunsyu Seijin Kango 1 (Collection of Papers in Adult Nursing no. 1); Japanese Nursing Association: Tokyo, Japan, 2006; Volume 37, pp. 76–78. (In Japanese) [Google Scholar]
- Shimada, Y.; Chida, S.; Matsunaga, T.; Itoi, E. Igensei Fuku-Shinkei Mahi ni taisuru Rehabilitation (Rehabilitation of iatrogenic accessory nerve paralysis). Bessatsu Seikei-Geka Orthop. Surg. Suppl. Vol. 2006, 49, 222–227. (In Japanese) [Google Scholar]
- Uchida, N.; Nagata, T.; Ito, K.; Hara, Y. Lymphedema following breast cancer operation and changes in lifestyle. Toyota J. Med. 2009, 19, 88–92. (In Japanese) [Google Scholar]
- Ozaki, M. The present situation of postoperative return to work in colorectal cancer patients based on clinical background. Jpn. J. Occup. Med. Traumatol. 2013, 61, 372–376. (In Japanese) [Google Scholar]
- Suzuki, T.; Itou, K. Social rehabilitation of gastric/colon cancer patients after laparoscopic surgery. Jpn. J. Occup. Med. Traumatol. 2014, 62, 382–387. (In Japanese) [Google Scholar]
- Ito, H.; Hozawa, A.; Yamashita, H.; Kubota, I.; Nemoto, K.; Yoshioka, T.; Kayama, T.; Murakami, M. Employment status among non-retired cancer survivors in Japan. Eur. J. Cancer Care Engl. 2015, 24, 718–723. [Google Scholar] [CrossRef]
- Nitta, K.; Egawa, C.; Okishiro, M.; Kusama, H.; Takeda, Y.; Kato, T.; Tamura, S.; Takatsuka, Y.; Hirooka, T.; Kano, S.; et al. The working situation of the breast cancer patient according to the treatment. Jpn. J. Occup. Med. Traumatol. 2015, 63, 276–283. (In Japanese) [Google Scholar]
- Matsuda, Y.; Tanaka, K.; Watanabe, Y.; Sato, I.; Tomuro, M. The actual employment conditions of cancer survivors living in the Tohoku region who are visiting the outpatient department after being diagnosed with cancer. J. Jpn. Soc. Cancer Nurs. 2015, 29, 73–78. (In Japanese) [Google Scholar]
- Endo, M.; Haruyama, Y.; Takahashi, M.; Nishiura, C.; Kojimahara, N.; Yamaguchi, N. Returning to work after sick leave due to cancer: A 365-day cohort study of Japanese cancer survivors. J. Cancer Surviv. 2016, 10, 320–329. [Google Scholar] [CrossRef]
- Shionoya, M.; Kurobe, A.; Kitazawa, S.; Komatsu, M.; Minagawa, M.; Hara, M.; Okamura, Y.; Kobayashi, A.; Naito, I.; Miyashita, Y.; et al. Gairai Kagakuryohou wo Ukeru Gan Kanja no Shindango no Syurou Joukyou no Henka nikannsuru Jittai Chosa–Gan Chiryo to Syurou no Ryouritsu niokeru Kadai–(Changes in working situations after cancer diagnosis of cancer patients taking chemotherapy at outpatient department–Agenda for achieving cancer treatment return to work in a compatible way–). Med. J. Nagano Munic. Hosp. 2016, 1, 59–65. (In Japanese) [Google Scholar]
- Nakamura, K.; Haraga, J.; Nishida, T.; Omichi, C.; Haruma, T.; Kusumoto, T.; Seki, N.; Masuyama, H.; Hiramatsu, Y. Fujinka Gan Chiryo-go niokeru Syuro Seikatsu no Genjo (Working statuses of patients with gynecological cancer after the operation). Sanfujinka no Jissai Obstet. Gynecol. Pract. 2017, 66, 211–215. (In Japanese) [Google Scholar]
- Takahara, Y.; Akahane, K.; Wakayama, H.; Murota, K.; Tagomori, K.; Matsuura, M.; Sasaki, T.; Kimata, T.; Hatakeyama, K.; Yamamuro, O. A survey of the working situation of cancer patients during chemotherapy and an effort to build a work support system. Jpn. J. Cancer Clin. 2017, 63, 347–353. (In Japanese) [Google Scholar]
- Tomita, M.; Takahashi, M.; Tagaya, N.; Kakuta, M.; Aoki, M.; Kai, I.; Muto, T. Factors associated with changes in employment status of women after breast cancer diagnosis. Jpn. J. Breast Cancer 2017, 32, 519–529. (In Japanese) [Google Scholar]
- Arfi, A.; Baffert, S.; Soilly, A.L.; Huchon, C.; Reyal, F.; Asselain, B.; Neffati, S.; Rouzier, R.; Héquet, D. Determinants of return at work of breast cancer patients: Results from the OPTISOINS01 French prospective study. BMJ Open 2018, 8, e020276. [Google Scholar]
- Roelen, C.A.; Koopmans, P.C.; Schellart, A.J.; van der Beek, A.J. Resuming work after cancer: A prospective study of occupational register data. J. Occup. Rehabil. 2011, 21, 431–440. [Google Scholar] [CrossRef]
- Short, P.F.; Vargo, M.M. Responding to employment concerns of cancer survivors. J. Clin. Oncol. 2006, 24, 5138–5141. [Google Scholar] [CrossRef]
- Nachreiner, N.M.; Dagher, R.K.; McGovern, P.M.; Baker, B.A.; Alexander, B.H.; Gerberich, S.G. Successful return to work for cancer survivors. AAOHN J. 2007, 55, 290–295. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Krause, N.; Dasinger, L.K.; Neuhauser, F. Modified Work and Return to Work: A Review of the Literature. J. Occup. Rehabil. 1998, 8, 113–139. [Google Scholar] [CrossRef]
- Choi, K.S.; Kim, E.J.; Lim, J.H.; Kim, S.G.; Lim, M.K.; Park, J.G.; Park, E.C. Job loss and reemployment after a cancer diagnosis in Koreans - a prospective cohort study. Psychooncology 2007, 16, 205–213. [Google Scholar] [CrossRef]
- Johnsson, A.; Fornander, T.; Olsson, M.; Nystedt, M.; Johansson, H.; Rutqvist, L.E. Factors associated with return to work after breast cancer treatment. Acta Oncol. 2007, 46, 90–96. [Google Scholar] [CrossRef] [Green Version]
- de Boer, A.G.; Verbeek, J.H.; Spelten, E.R.; Uitterhoeve, A.L.; Ansink, A.C.; de Reijke, T.M.; Kammeijer, M.; Sprangers, M.A.; van Dijk, F.J. Work ability and return-to-work in cancer patients. Br. J. Cancer 2008, 98, 1342–1347. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; Fornander, T.; Rutqvist, L.E.; Vaez, M.; Alexanderson, K.; Olsson, M. Predictors of return to work ten months after primary breast cancer surgery. Acta Oncol. 2009, 48, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.F.; Hankins, M.; Rixon, L.; Eaton, E.; Grunfeld, E.A. Distinct work-related, clinical and psychological factors predict return to work following treatment in four different cancer types. Psychooncology 2013, 22, 659–667. [Google Scholar] [CrossRef]
- Wayant, C.; Scheckel, C.; Hicks, C.; Nissen, T.; Leduc, L.; Som, M.; Vassar, M. Evidence of selective reporting bias in hematology journals: A systematic review. PLoS ONE 2017, 12, e0178379. [Google Scholar] [CrossRef]
- Park, J.H.; Park, E.C.; Park, J.H.; Kim, S.G.; Lee, S.Y. Job loss and re-employment of cancer patients in Korean employees: A nationwide retrospective cohort study. J. Clin. Oncol. 2008, 26, 1302–1309. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.H.; Kim, S.G. Effect of cancer diagnosis on patient employment status: A nationwide longitudinal study in Korea. Psychooncology 2009, 18, 691–699. [Google Scholar] [CrossRef]

| First Author (Publication Year) | Study Design and Setting | At Cancer Diagnosis | (1) Cancer Site, (2) Stage, (3) Treatment | At Return to Work | |
|---|---|---|---|---|---|
| (1) Number, (2) Sex, (3) Age | Employment Status | (1) Return-to-Work Rate, (2) Employment Status among Those Who Returned to Work, (3) Duration between Cancer Diagnosis and Assessment of Return to Work | |||
| Okumura (2006) [11] | C, H | (1) 42, (2) Female only, (3) ‡ Mean (SD): 53.8 (23.5), range: 27–74 | NA | (1) Female genitals, (2) NA, (3) ‡ Ope: 58%, Ope+Che and/or Rad: 42% | (1) 95.2%, (2) Working style was reported. Standing work: 60%, sedentary work: 33%, (3) Return to work <1 month after Ope: 25%, <3 months: 35%, <6 months: 20% |
| Shimada (2006) [12] | C, H | (1) 13, (2) ‡ Male: 61%, (3) ‡ Mean 58, range: 39–73 | NA | (1) Head/neck, (2) NA, (3) Ope (RND): 100% | (1) 53.8%, (2) Former work, (3) NA |
| Uchida (2009) [13] | C, H | (1) 32, (2) Female only, (3) ‡ Mean: 52, range: 28–73 | NA | (1) Breast, (2) NA, (3) Ope (ALND): 100%, Che: 0% | (1) 56.3%, (2) NA, (3) Mean: 23 months, range 4–38 |
| Ozaki (2013) [14] | C, H | (1) 164, (2) Male: 65%, (3) ‡ 50–59: 31%, 60–69: 53% | NA | (1) Colon, (2) ‡ I: 14%, II: 28%, III: 30%, IV: 20%, NA: 8%, (3) ‡ LT: 78%, LS: 17% | (1) Total: 70.1%, † 51.8%, (2) All occupations including homemaker, (3) Range: 100–2000 days |
| Suzuki (2014) [15] | C, H | (1) 78, (2) ‡ Male: 73%/51% (stomach/colon), (3) Mean: 56.2/57.0 (stomach/colon) | NA | (1) 54%/46% (stomach/colon), (2) NA, (3) LT: 43%/33%, LS: 57%/67% (stomach/colon) | (1) 42.9%/80.6% (stomach/colon), (2) NA, (3) 1 month after Ope |
| Ito (2015) [16] | C, H | (1) 260, (2) Male: 50%, (3) Mean (SD): 54.9 (8.3) | Regularly employed: 48%, self-employed: 25%, non-regularly employed: 15% | (1) NA, (2) Early: 34%, advanced: 54%, NA: 12%, (3) Ope: 72%, Che: 63%, Rad: 36% | (1) Total: 74.6%, (2) Regularly employed: 47%, self-employed: 27%, non-regularly employed: 15%, (3) Mean (SD): 4.2 (3.5) years |
| Nitta (2015) [17] (new onset, Ope+Che) | C, H | (1) 17, (2) Female only, (3) ‡ Median: 54, range: 31–75 | Regularly employed: 41%, part-time: 29%, self-employed: 24% | (1) Breast, (2) ‡ I: 17%, II: 70%, III: 13%, (3) ‡ Ope+Che (including MTT): 100% | (1) 41.2%, (2) Former work: 86%, (3) NA |
| Nitta (2015) [17] (new onset, Ope+ET) | C, H | (1) 17, (2) Female only, (3) ‡ Median: 55, range: 34–74 | Regularly employed: 35%, part-time: 41%, self-employed: 18% | (1) Breast, (2) ‡ I: 26%, II: 59%, III: 7%, (3) ‡ Ope+ET: 100% | (1) 64.7%, (2) Former work: 100%, (3) NA |
| Nitta (2015) [17] (recurrence, Che) | C, H | (1) 11, (2) Female only, (3) ‡ Median: 51, range: 33–67 | Regularly employed: 27%, part-time: 45%, self-employed: 18% | (1) Breast, (2) ‡ I: 10%, II: 45%, III: 20%, NA: 25%, (3) ‡ Ope: 95%, Che (including MTT): 100% | (1) 45.5%, (2) Former work: 60%, (3) Median: 51 months, range: 1–132 months |
| Nitta (2015) [17] (recurrence, ET) | C, H | (1) 10, (2) Female only, (3) ‡ Median: 55, range: 36–78 | Regularly employed: 20%, part-time: 50%, self-employed: 20% | (1) Breast, (2) ‡ I: 27%, II: 36%, III: 23%, NA: 14%, (3) ‡ Ope: 82%, ET: 100% | (1) 70.0%, (2) Former work: 71%, (3) Median: 97 months, range: 3–180 months |
| Nitta (2015) [17] (follow-up) | C, H | (1) 12, (2) Female only, (3) ‡ Median: 51, range: 33–67 | Regularly employed: 50%, part-time: 17%, temporary: 25% | (1) Breast, (2) ‡ I: 55%, II: 27%, III: 14%, (3) ‡ Ope: 100%, Che: 50%, ET: 50%, Rad: 73% | (1) 91.7%, (2) Former work: 73%, (3) Median: 72 months, range: 5–120 months |
| Matsuda (2015) [18] | C, H | (1) 62, (2) ‡ Male: 42%, (3) ‡ 30–39: 11%, 40–49: 17%, 50–59: 32%, 60–69: 30% | Regularly employed: 39%, self-employed: 23%, homemaker: 19%, part-time: 10%, civil servant: 8% | (1) ‡ Breast: 24%, lung: 20%, stomach: 11%, uterine: 9%, colon: 5%, other: 32%, (2) ‡ I: 24%, II: 9%, III: 14%, IV: 20%, NA: 33%, (3) ‡ Ope: 58%, Che: 83%, Rad: 23% | (1) Total: 83.9%, † 59.7%, (2) Regularly employed: 33%, self-employed: 17%, homemaker: 29%, part-time: 6%, civil servant: 10%, (3) ‡ <2 years: 65%, 2–6 years: 20%, 7–9 years: 11%, 10+ years: 3% |
| Endo (2016) [19] | P, W | (1) 1278, (2) Male: 81%, (3) Mean: 51.9 | Employed by large-scale company: 100% | (1) Stomach: 22%, lung: 13%, intestine: 11%, breast: 8%, female genitals: 5%, (2) NA, (3) NA | (1) Total: 80.7%, (2) Former company, (3) 365 days |
| Shionoya (2016) [20] | C, H | (1) 73, (2) Male: 42%, (3) 40–49: 8%, 50–59: 25%, 60–69: 44%, 70–79: 16% | Self-employed: 34%, regularly employed: 32%, part-time: 26%, temporary: 8% | (1) Breast: 27%, colon: 25%, female genitals: 12%, lung: 10%, liver/GB/pancreas: 10%, (2) NA, (3) Che at outpatient clinic: 100% | (1) Total: 63.0%, male: 81.8%, female: 47.5%, age 40–49: 50.0%, 50–59: 33.3%, 60–69: 71.9%, 70–79: 91.7%, self-employed: 88.0%, regularly employed: 73.9%, part-time: 15.8%, temporary: 66.7%, (2) NA, (3) NA |
| Nakamura (2017) [21] | C, H | (1) 213, (2) Female only, (3) Median: 45/48/48 (CC/EC/OC) | Part-time: 43%/42%/33%, regularly employed: 33%/34%/36%, self-employed: 16%/12%/21%, civil servant: 8%/12%/9% (CC/EC/OC) | (1) 53%/31%/15% (CC/EC/OC), (2) Early (I and II): 87%/90%/76%, advanced (III and IV): 13%/10%/24% (CC/EC/OC), (3) Ope: 38%/66%/21% (CC/EC/OC), Ope+Che/Rad: 36% (CC), Che/Rad: 26% (CC), Ope+Che: 34%/79% (EC/OC) | (1) 85.0%/85.0%/88.1%/78.8% (Total/CC/EC/OC), (2) Former worksite: 82%/85%/100% (CC/EC/OC), (3) 1 year or more following start of treatment |
| Takahara (2017) [22] | C, H | (1) 61, (2) ‡ Male: 36%, (3) ‡ (Male) 50–59: 25%, 60–69: 72%, (Female) 40–49: 35%, 50–59: 30%, 60–69: 30% | Regularly employed: 48%, non-regularly employed: 33%, self-employed: 20% | (1) ‡ Breast: 27%, colon: 24%, lung: 13%, lymphoma: 9%, female genitals: 8%, (2) ‡ I: 10%, II: 17%, III: 25%, IV: 31%, NA: 17%, (3) Che at outpatient clinic: 100% | (1) Total: 63.9%, regularly employed: 69.0%, non-regularly employed: 55.0%, self-employed: 66.7%, (2) Regularly employed: 51%, non-regularly employed: 28%, self-employed: 21%, (3) ‡ <2 years: 51%, 2–4 years: 28%, 4–6 years: 10%, 7+ years: 11% |
| Tomita (2017) [23] | C, H | (1) 84, (2) Female only, (3) Mean (SD): 55.4 (8.7), range: 31–77 (at time of survey) | Regularly employed: 67%, part-time: 33% | (1) Breast, (2) I: 36%, II: 45%, III: 13%, IV: 6%, (3) Ope: 98%, Che: 57%, HT: 74%, Rad: 67% | (1) 70.2%, (2) Regularly employed: 56%, part-time: 44% (3) Mean (SD): 62 (40) months, range: 10–201 months |
| First Author (Publication Year) | Risk of Bias Assessment Tool for Non-Randomized Studies (RoBANS) Domain and Risk of Bias | |||||
|---|---|---|---|---|---|---|
| 1. Selection of Participants (Selection Bias) | 2. Confounding Variables (Selection Bias) | 3. Measurement of Exposure (Cancer Diagnosis) (Performance Bias) | 4. Blinding of Outcome (Return to Work) Assessment (Detection Bias) | 5. Incomplete Outcome Data (Attrition Bias) | 6. Selective Outcome Reporting (Reporting Bias) | |
| Okumura (2006) [11] | High | High | Low | Low | High | Low |
| Shimada (2006) [12] | High | High | Low | Unclear | Unclear | High |
| Uchida (2009) [13] | High | High | Low | Low | High | High |
| Ozaki (2013) [14] | High | High | Low | Low | High | High |
| Suzuki (2014) [15] | High | High | Low | Low | Unclear | High |
| Ito (2015) [16] | High | High | Low | Low | High | Low |
| Nitta (2015) [17] | High | Low | Low | Low | Unclear | High |
| Matsuda (2015) [18] | High | Low | Low | Low | High | Low |
| Endo (2016) [19] | Low | High | Low | Low | Low | Low |
| Shionoya (2016) [20] | High | High | Low | Low | High | High |
| Nakamura (2017) [21] | High | Low | Low | Low | High | Low |
| Takahara (2017) [22] | High | Low | Low | Low | High | Low |
| Tomita (2017) [23] | High | Low | Low | Low | High | Low |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, A.; Fujisawa, A.; Kawada, K.; Yatsuya, H. Recent Status and Methodological Quality of Return-to-Work Rates of Cancer Patients Reported in Japan: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1461. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16081461
Ota A, Fujisawa A, Kawada K, Yatsuya H. Recent Status and Methodological Quality of Return-to-Work Rates of Cancer Patients Reported in Japan: A Systematic Review. International Journal of Environmental Research and Public Health. 2019; 16(8):1461. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16081461
Chicago/Turabian StyleOta, Atsuhiko, Akiko Fujisawa, Kenji Kawada, and Hiroshi Yatsuya. 2019. "Recent Status and Methodological Quality of Return-to-Work Rates of Cancer Patients Reported in Japan: A Systematic Review" International Journal of Environmental Research and Public Health 16, no. 8: 1461. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16081461





