The Dose of Fungal Aerosol Inhaled by Workers in a Waste-Sorting Plant in Poland: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Sampling and Analysis Methods
2.3. Identification of Selected Fungi
- (1)
- Growing a pure fungus culture on a Petri dish with MEA 2% until enough conidiation is present to prepare a suspension;
- (2)
- Swabbing the conidia on the surface of the agar plate and suspending to a specified density in FF inoculating fluid;
- (3)
- Adding 100 μl of suspension into each well of the FF MicroPlate;
- (4)
- Incubation of the FF MicroPlate at 26 °C for 24–96 hours;
- (5)
- Reading the MicroPlates using the Biolog MicroStation™.
2.4. Calculation of the Fungal Waste-Sorting Plant Exposure Dose (FWSPED)
3. Results and Discussion
3.1. Fungal Waste-Sorting Plant Exposure Dose (FWSPED)
3.2. Identification of Fungal Aerosol
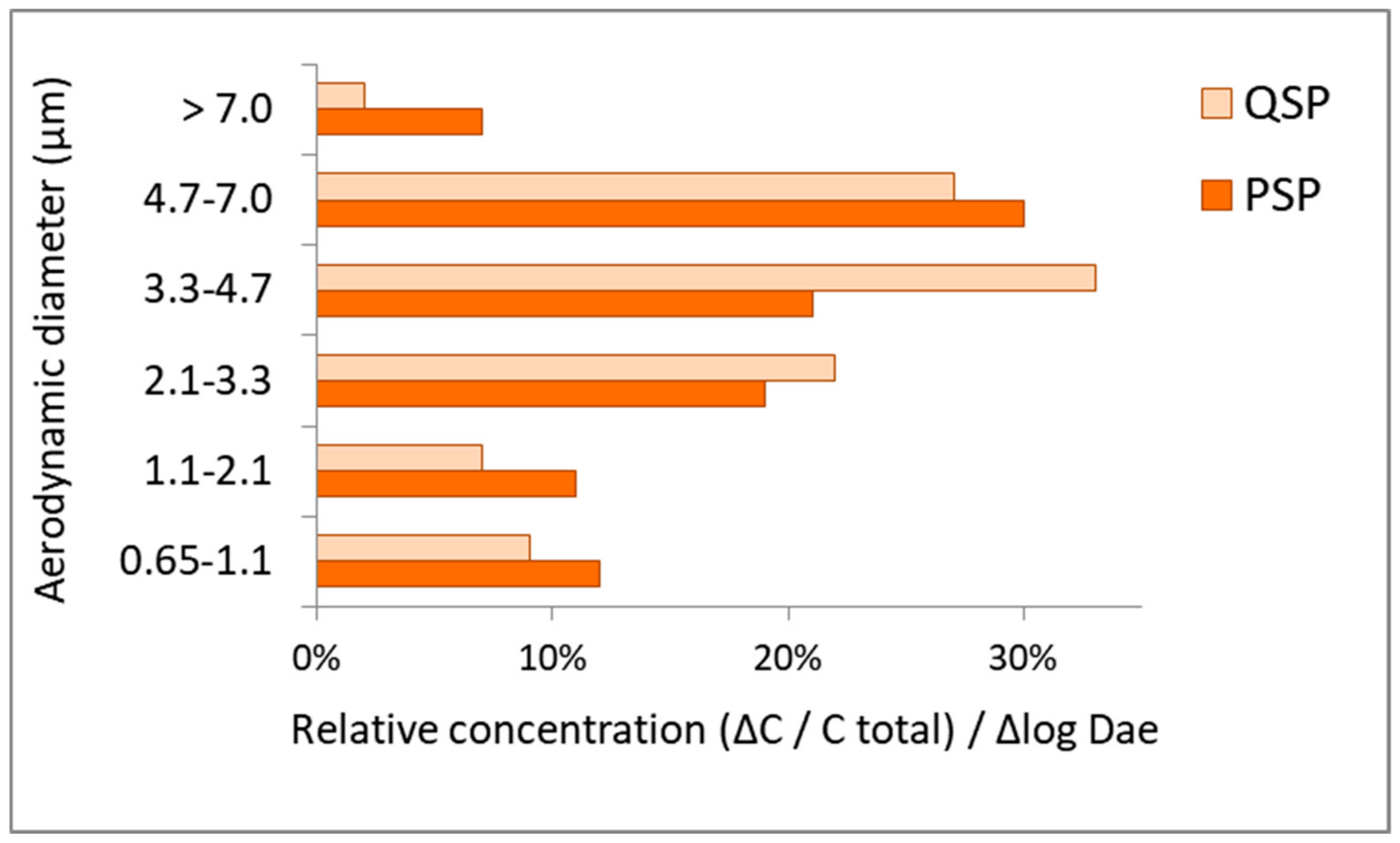
3.3. Particle Size Distribution (PSD) of Isolated Fungi
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Babatola, S.S. Global burden of diseases attributable to air pollution. J. Public Health Afr. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Effects Institute. State of Global Air 2019; Special Report; Health Effects Institute: Boston, MA, USA, 2019. [Google Scholar]
- Gola, M.; Settimo, G.; Capolongo, S. Indoor Air Quality in Inpatient Environments: A Systematic Review on Factors that Influence Chemical Pollution in Inpatient Wards. J. Healthc. Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wéry, N. Bioaerosols from composting facilities—A review. Front. in Cell. Infect. Microbiol. 2014, 4, 42. [Google Scholar]
- Binion, E.; Gutberlet, J. The effects of handling solid waste on the wellbeing of informal and organized recyclers: A review of the literature. Int. J. Occup. Environ. Health 2012, 18, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. In Annals of Occupational Hygiene; Oxford University Press: Oxford, UK, 2003; Volume 47, pp. 187–200. [Google Scholar]
- Hoffmeyer, F.; van Kampen, V.; Taeger, D.; Deckert, A.; Rosenkranz, N.; Kaßen, M.; Schantora, A.L.; Brüning, T.; Raulf, M.; Bünger, J. Prevalence of and relationship between rhinoconjunctivitis and lower airway diseases in compost workers with current or former exposure to organic dust. Ann. Agric. Environ. Med. 2014, 21, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anedda, E.; Carletto, G.; Gilli, G.; Traversi, D. Monitoring of Air Microbial Contaminations in Different Bioenergy Facilities Using Cultural and Biomolecular Methods. Int. J. Environ. Res. Public Health 2019, 16, 2546. [Google Scholar] [CrossRef] [Green Version]
- Morgado Gamero, W.B.; Ramírez, M.C.; Parody, A.; Viloria, A.; López, M.H.A.; Kamatkar, S.J. Concentrations and size distributions of fungal bioaerosols in a municipal landfill. In Data Mining and Big Data. LNCS; Tan, Y., Shi, Y., Tang, Q., Eds.; Springer: Basel, Switzerland, 2018. [Google Scholar] [CrossRef]
- Fang, Z.G.; Ouyang, Z.Y.; Zheng, H.; Wang, X.K. Concentration and size distribution of culturable airborne microorganisms in outdoor environments in Beijing, China. Aerosol Sci. Technol. 2008, 42, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Al Maghlouth, A.; Al Yousef, Y.; Al-Bagieh, N.H. Qualitative and quantitative analysis of microbial aerosols in selected areas within the College of Dentistry, King Saud University. Quintessence Int. 2007, 38, 222–228. [Google Scholar]
- Tolabi, Z.; Alimohammadi, M.; Hassanvand, M.S.; Nabizadeh, R.; Soleimani, H.; Zarei, A. The investigation of type and concentration of bio-aerosols in the air of surgical rooms: A case study in Shariati hospital, Karaj. MethodsX 2019, 6, 641–650. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J. Bacterial and Fungal Aerosols in Rural Nursery Schools in Southern Poland. Atmosphere 2016, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Brągoszewska, E.; Biedroń, I.; Kozielska, B.; Pastuszka, J.S. Microbiological indoor air quality in an office building in Gliwice, Poland: Analysis of the case study. Air Qual. Atmos. Health 2018, 11, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Phalen, R.F.; Mendez, L.B. Dosimetry considerations for animal aerosol inhalation studies. Biomarkers 2009, 14, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.; Lizończyk, K.; Desta, Y. Assessment of Bacterial Aerosol in a Preschool, Primary School and High School in Poland. Atmosphere 2018, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Černá, K.; Wittlingerová, Z.; Zimová, M.; Janovský, Z. Exposure to airborne fungi during sorting of recyclable plastics in waste treatment facilities. Med. Pracy 2017, 68, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malta-Vacas, J.; Viegas, S.; Sabino, R.; Viegas, C. Fungal and microbial volatile organic compounds exposure assessment in a waste sorting plant. J. Toxicol. Environ. Health Part A 2012, 75, 1410–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtinen, J.; Tolvanen, O.; Nivukoski, U.; Veijanen, A.; Hänninen, K. Occupational hygiene in terms of volatile organic compounds (VOCs) and bioaerosols at two solid waste management plants in Finland. Waste Manag. 2013, 33, 964–973. [Google Scholar] [CrossRef]
- Frankel, M.; Bekö, G.; Timm, M.; Gustavsen, S.; Hansen, E.W.; Madsen, A.M. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl. Environ. Microbiol. 2012, 78, 8289–8297. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, O.M.; Breum, N.O.; Ebbehøj, N.; Hansen, Å.M.; Ivens, U.I.; van Lelieveld, D.; Malmros, P.; Matthiasen, L.; Nielsen, B.H.; Nielsen, E.M.; et al. Sorting and recycling of domestic waste. Review of occupational health problems and their possible causes. Sci. Total Environ. 1995, 168, 33–56. [Google Scholar] [CrossRef]
- Eduard, W.; Heederik, D.; Duchaine, C.; Green, B.J. Bioaerosol exposure assessment in the workplace: The past, present and recent advances. J. Environ. Monit. 2012, 14, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Fischer, G.; Dott, W. Relevance of airborne fungi and their secondary metabolites for environmental, occupational and indoor hygiene. Arch. Microbiol. 2003, 179, 75–82. [Google Scholar] [CrossRef]
- Nazaroff, W.W. Indoor bioaerosol dynamics. Indoor Air 2016, 26, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Zamfir, M.; Gerstner, D.G.; Walser, S.M.; Bünger, J.; Eikmann, T.; Heinze, S.; Kolk, A.; Nowak, D.; Raulf, M.; Sagunski, H.; et al. A systematic review of experimental animal studies on microbial bioaerosols: Dose-response data for the derivation of exposure limits. Int. J. Hyg. Environ. Health 2019, 222, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Littlewood, E.; Douglas, P.; Robertson, S.; Gant, T.W.; Hansell, A.L. Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: A systematic review of occupational and community studies. J. Toxicol. Environ. Health Part B Crit. Rev. 2015, 18, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of Health dated 22 April 2005 (Journal of Laws of 2005, No. 81, item 716, as amended and Journal of Laws 2008, No. 48, item 288). in Polish.
- Brągoszewska, E.; Biedroń, I.; Hryb, W. Air Quality and Potential Health Risk Impacts of Exposure to Bacterial Aerosol in a Waste Sorting Plant Located in the Mountain Region of Southern Poland, Around Which There Are Numerous Rural Areas. Atmosphere 2019, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Nevalainen, A.; Willeke, K.; Liebhaber, F.; Pastuszka, J.S.; Burge, H.; Henningson, E. Bioaerosol sampling. In Aerosol Measurement: Principles, Techniques and Applications; Willeke, K., Baron, P., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1993; pp. 471–492. [Google Scholar]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471. [Google Scholar]
- PN-EN 12322. In Vitro Diagnostic Medical Devices. Culture Media for Microbiology. Performance Criteria for Culture Media. 2005. Available online: https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/iv-diagnostic-medical-devices_en (accessed on 10 November 2019).
- ISO 11133. Microbiology of Food, Animal Feed and Water—Preparation, Production, Storage and Performance Testing of Culture Media. Available online: https://www.iso.org/standard/53610.html (accessed on 10 November 2019).
- Brągoszewska, E.; Biedroń, I. Indoor Air Quality and Potential Health Risk Impacts of Exposure to Antibiotic Resistant Bacteria in an Office Rooms in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2604. [Google Scholar] [CrossRef] [Green Version]
- Raper, K.; Fennel, D. The genus Aspergillus; Williams & Wilkins Baltimore: Baltimore, MD, USA, 1965. [Google Scholar]
- Klaus, H.; Domsch, W.; Gams, W.; Anderson, T. Compendium of Soil Fungi. Eur. J. Soil Sci. 2008, 59, 1007. [Google Scholar]
- de Hoog, G.S.; Queiroz-Telles, F.; Haase, G.; Fernandez-Zeppenfeldt, G.; Angelis, A.A.; van den Ende, A.H.G.G.; Matos, T.; Peltroche-Llacsahuanga, H.; Pizzirani-Kleiner, A.A.; Rainer, J.; et al. Black fungi: Clinical and pathogenic approaches. Med. Mycol. 2000, 38, 243–250. [Google Scholar] [CrossRef]
- U.S. EPA. Exposure Factors Handbook; Environmental Protection Agency: Washington, DC, USA, 2011.
- Ott, W.R.; Steinemann, A.C.; Wallace, L.A. Exposure Analysis; CRC Press: London, UK, 2006. [Google Scholar]
- Johnson-Restrepo, B.; Kannan, K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009, 76, 542–548. [Google Scholar] [CrossRef]
- Mostafa, D.M. Exposure Dose of Bacteria and Fungi in a Public Primary School in Beni Suef, Upper Egypt. J. Adv. Biol. 2019. [Google Scholar] [CrossRef]
- Bragoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and size distribution of culturable bacteria in ambient air during spring and winter in Gliwice: A typical urban area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Brągoszewska, E.; Pastuszka, J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia 2018, 34, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Kozajda, A.; Szadkowska-Stanczyk, I. Selected health complains, allergic diseases, hygiene behaviors and knowledge of biohazards among workers of waste sorting plants. Med. Pracy 2009, 60, 491–499. [Google Scholar]
- Reinthaler, F.F.; Haas, D.; Feierl, G.; Schlacher, R.; Pichler-Semmelrock, F.P.; Köck, M.; Wüst, G.; Feenstra, O.; Marth, E. Comparative investigations of airborne culturable microorganisms in selected waste treatment facilities and in neighbouring residential areas. Int. J. Hyg. Environ. Med. 1999, 202, 1–17. [Google Scholar] [CrossRef]
- Würtz, H.; Breum, N.O. Exposure to microorganisms during manual sorting of recyclable paper of different quality. Ann. Agric. Environ. Med. 1997, 4, 129–136. [Google Scholar]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Viegas, C.; Gomes, A.Q.; Abegão, J.; Sabino, R.; Graça, T.; Viegas, S. Assessment of fungal contamination in waste sorting and incineration—Case study in Portugal. J. Toxicol. Environ. Health Part A 2014, 77, 57–68. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Jun, S.C.; Han, K.H.; Hong, S.B.; Yu, J.H. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2017; Volume 100, pp. 161–202. [Google Scholar]
- Barros, G.; Torres, A.; Chulze, S. Aspergillus flavus population isolated from soil of Argentina’s peanut-growing region. Sclerotia production and toxigenic profile. J. Sci. Food Agric. 2005, 85, 2349–2353. [Google Scholar] [CrossRef]
- Zhang, C.; Selvaraj, J.N.; Yang, Q.; Liu, Y. A survey of aflatoxin-producing Aspergillus sp. from peanut field soils in four agroecological zones of China. Toxins 2017, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Olatunde Farombi, E. Aflatoxin contamination of foods in developing countries: Implications for hepatocellular carcinoma and chemopreventive strategies. Afr. J. Biotechnol. 2006, 5, 1–14. [Google Scholar]
- Campbell, K.W.; White, D.G. Evaluation of Corn Genotypes for Resistance to Aspergillus Ear Rot, Kernel Infection, and Aflatoxin Production. Plant Dis. 1995. [Google Scholar] [CrossRef]
- Viegas, S.; Veiga, L.; Figueiredo, P.; Almeida, A.; Carolino, E.; Viegas, C. Assessment of workers’ exposure to aflatoxin B1 in a Portuguese waste industry. Ann. Occup. Hyg. 2015, 59, 173–181. [Google Scholar] [PubMed] [Green Version]
- Benassi, V.M.; de Lucas, R.C.; Michelin, M.; Jorge, J.A.; Terenzi, H.F.; P Polizeli, M.D.L.T.D. Production and action of an Aspergillus phoenicis enzymatic pool using different carbon sources. Braz. J. Food Technol. 2012, 15, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Dixon, D.M.; Polak-Wyss, A. The medically important dematiaceous fungi and their identification: Medizinisch wichtige Schwärzepilze und ihre Identifizierung. Mycoses 1991, 34, 1–18. [Google Scholar] [CrossRef]
- AlMatar, M.; Makky, E.A. Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech 2016, 6, 4. [Google Scholar] [CrossRef]
- Mushimiyimana, I.; Tallapragada, P. Optimization of process parameters for biosynthesis of cellulase by Cladosporium Cladosporioides using agro wastes. Int. J.Pharma Bio Sci. 2013, 4, 4. [Google Scholar]
- Li, L.; Li, Q.; Liu, Z.; Wu, H. Immunological analysis and mass spectrometry identification of the major allergen from Cladosporium cladosporioides. J. Hyg. Res. 2008, 37, 50–52. [Google Scholar]
- Barcus, A.L.; Burdette, S.D.; Herchline, T.E. Intestinal invasion and disseminated disease associated with Penicillium chrysogenum. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Howard, D.H. (Ed.) Pathogenic Fungi in Humans and Animals, 2nd ed.; Mycology Series; Marcel Dekker: New York, NY, USA, 2003; Volume 16, ISBN 0-8247-0683-8. [Google Scholar]
- Di Filippo, P.; Pomata, D.; Riccardi, C.; Buiarelli, F.; Perrino, C. Fungal contribution to size-segregated aerosol measured through biomarkers. Atmos. Environ. 2013, 64, 132–140. [Google Scholar] [CrossRef]
- Yang, C.S.; Johanning, E.; Li, D.W. Airborne Fungi and Mycotoxins. In Manual of Environmental Microbiology, 4th ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Bulski, K.; Frączek, K.; Chmiel, M.J. Microbiological air quality at municipal waste sorting plant. Ochr. Sr. Zasobow Nat. 2016, 27, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Hwang, G.B.; Jung, J.H.; Lee, D.H.; Lee, B.U. Generation characteristics of fungal spore and fragment bioaerosols by airflow control over fungal cultures. J. Aerosol Sci. 2010, 41, 319–325. [Google Scholar] [CrossRef]

| PSP | QSP | |
|---|---|---|
| Ventilation system | Undergoes a 20-fold air exchange every hour (h); the cabins have supply and exhaust ventilation, and the air is drawn away from the conveyor belts. | |
| Volume (m3) | 178 | 565 |
| Number of occupants | 6 | 10 |
| Indoor temperature (°C) | 18.3–21.4 | 17.1–19.4 |
| Indoor relative humidity (%) | 18–22 | 26–34 |
| Outdoor temperature (°C) Outdoor relative humidity (%) | 10.2–12.2 25.2–28 | |
| Waste-Sorting Plant (WSP) | ||
|---|---|---|
| Parameter | Short-Term Inhalation Rates by Activity Level/Minutes | |
| Activity Levels | Staff | Staff |
| (m3/min) | (hour) | |
| Sedentary/passive | 0.0048 | 2 |
| Light intensity | 0.013 | 2 |
| Moderate intensity | 0.028 | 2 |
| High intensity | 0.052 | 2 |
| Body weight (kg) | PSP 70; QSP 60 | |
| FWSPED—Waste-Sorting-Plant EX Dose (CFU/kg) | AVC—Average Concentration (CFU/m3) | |
|---|---|---|
| PSP | 193 | 8.1 ± 4.0 x 102 |
| QSP | 185 | 7.2 ± 3.1 x 102 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brągoszewska, E. The Dose of Fungal Aerosol Inhaled by Workers in a Waste-Sorting Plant in Poland: A Case Study. Int. J. Environ. Res. Public Health 2020, 17, 177. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17010177
Brągoszewska E. The Dose of Fungal Aerosol Inhaled by Workers in a Waste-Sorting Plant in Poland: A Case Study. International Journal of Environmental Research and Public Health. 2020; 17(1):177. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17010177
Chicago/Turabian StyleBrągoszewska, Ewa. 2020. "The Dose of Fungal Aerosol Inhaled by Workers in a Waste-Sorting Plant in Poland: A Case Study" International Journal of Environmental Research and Public Health 17, no. 1: 177. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17010177





