Motoric Cognitive Risk Syndrome Using Three-Item Recall Test and Its Associations with Fall-Related Outcomes: The Korean Frailty and Aging Cohort Study
Abstract
:1. Introduction
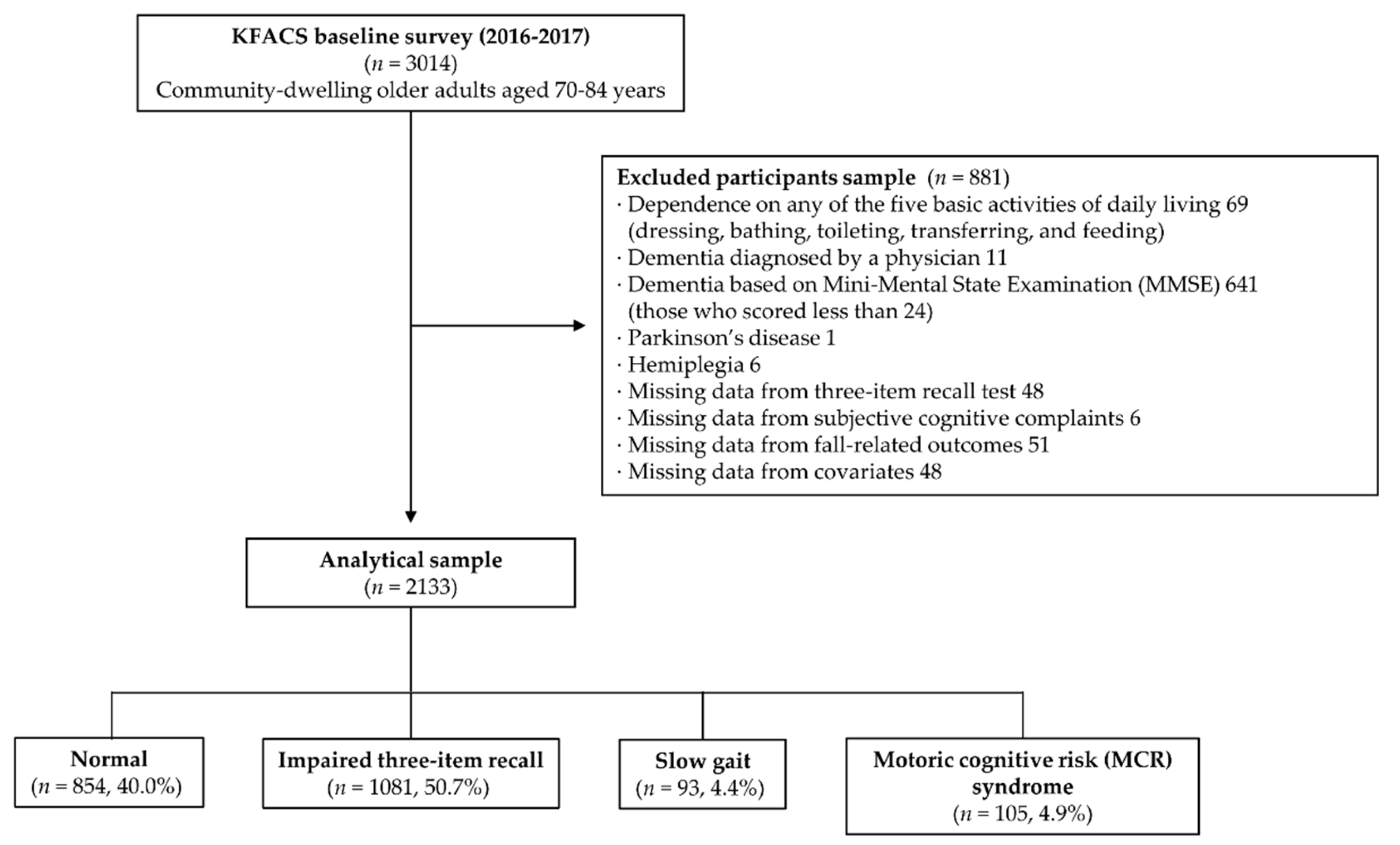
2. Materials and Methods
2.1. Study Population
2.2. Definitions of Motoric Cognitive Risk (MCR) Syndrome
2.2.1. Original MCR using Subjective Cognitive Complaints (SCCs)
2.2.2. New MCR using three-item recall (3IR)
2.3. Definitions of Fall-Related Outcomes
2.4. Measurements
2.5. Statistical Analyses
3. Results
3.1. Descriptive Characteristics of the Study Population
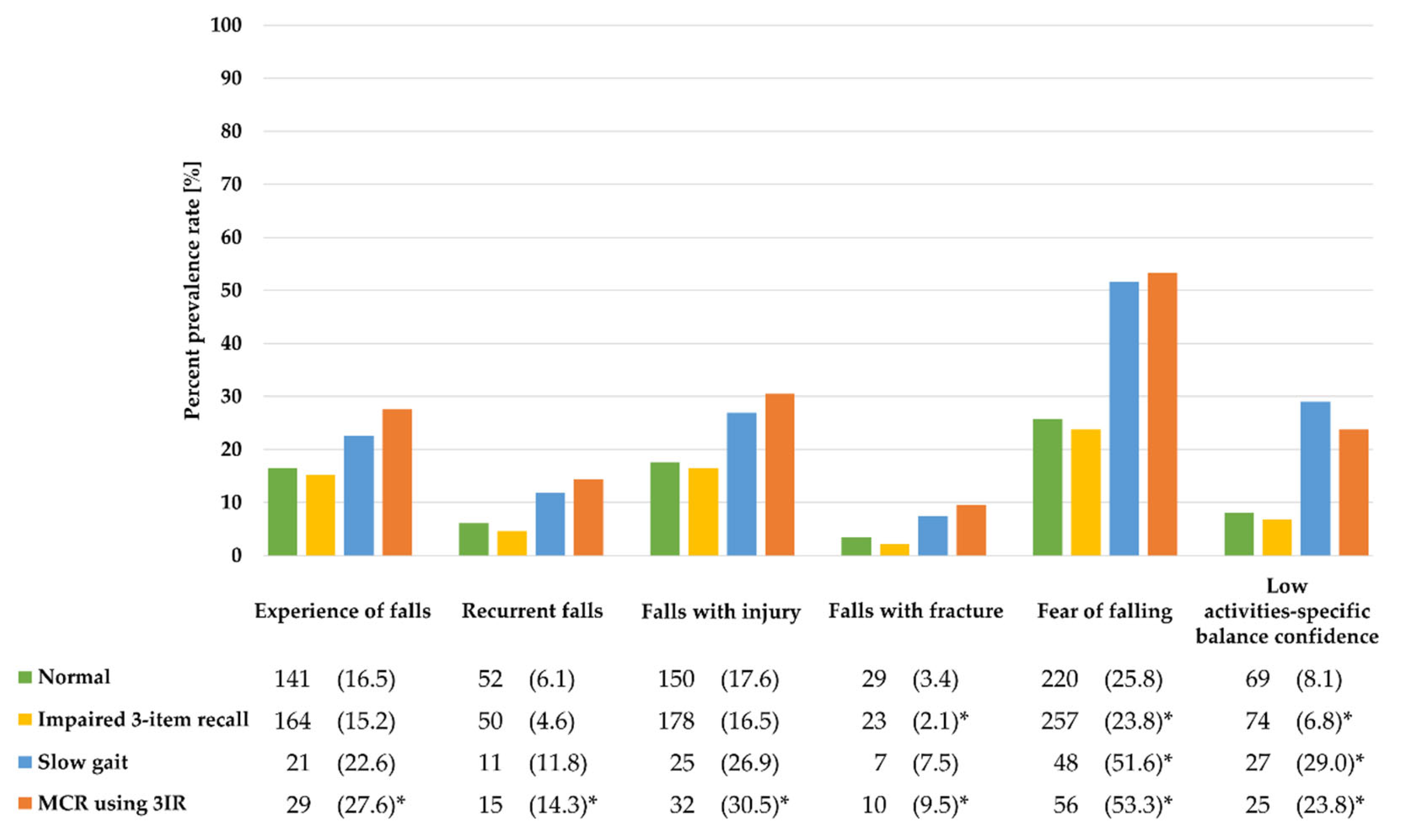
3.2. Associations of MCR using 3IR or MCR using SCCs with Fall-Related Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012, 60, 2127–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.A.; Verghese, J.; Zwerling, J.L. Cognition and gait in older people. Maturitas 2016, 93, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhetri, J.K.; Chan, P.; Vellas, B.; Cesari, M. Motoric Cognitive Risk Syndrome: Predictor of Dementia and Age-Related Negative Outcomes. Front. Med. 2017, 4, 166. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, H.; Allali, G.; Launay, C.P.; Barden, J.; Szturm, T.; Liu-Ambrose, T.; Chester, V.L.; Wong, C.H.; Beauchet, O.; Canadian Gait, C. Motoric cognitive risk syndrome, incident cognitive impairment and morphological brain abnormalities: Systematic review and meta-analysis. Maturitas 2019, 123, 45–54. [Google Scholar] [CrossRef]
- Meiner, Z.; Ayers, E.; Verghese, J. Motoric Cognitive Risk Syndrome: A Risk Factor for Cognitive Impairment and Dementia in Different Populations. Annals Geriatr. Med. Res. 2020, 24, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.A.; Schneider, J.A.; Buchman, A.S.; Barnes, L.L.; Boyle, P.A.; Wilson, R.S. Overview and findings from the rush Memory and Aging Project. Curr. Alzheimer Res. 2012, 9, 646–663. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Arvanitakis, Z.; Wilson, R.S. Overview and findings from the religious orders study. Curr. Alzheimer Res. 2012, 9, 628–645. [Google Scholar] [CrossRef] [Green Version]
- Verghese, J.; Annweiler, C.; Ayers, E.; Barzilai, N.; Beauchet, O.; Bennett, D.A.; Bridenbaugh, S.A.; Buchman, A.S.; Callisaya, M.L.; Camicioli, R. Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology 2014, 83, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Caramelli, P.; Beato, R.G. Subjective memory complaints and cognitive performance in a sample of healthy elderly. Dement. Neuropsychol. 2008, 2, 42. [Google Scholar] [CrossRef] [Green Version]
- Reid, L.M.; MacLullich, A.M. Subjective memory complaints and cognitive impairment in older people. Dement. Geriatr. Cognit. Dis. 2006, 22, 471–485. [Google Scholar] [CrossRef]
- Burmester, B.; Leathem, J.; Merrick, P. Subjective Cognitive Complaints and Objective Cognitive Function in Aging: A Systematic Review and Meta-Analysis of Recent Cross-Sectional Findings. Neuropsychol. Rev. 2016, 26, 376–393. [Google Scholar] [CrossRef]
- Sekhon, H.; Allali, G.; Beauchet, O. The association of anxio-depressive disorders and depression with motoric cognitive risk syndrome: Results from the baseline assessment of the Canadian longitudinal study on aging. Geroscience 2019, 41, 409–418. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Scott, D.; Kotowicz, M.A.; Nicholson, G.C. Validity of 12-Month Falls Recall in Community-Dwelling Older Women Participating in a Clinical Trial. Int. J. Endocrinol. 2015, 2015, 210527. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Min, J.Y.; Min, H.Y.; Lee, H.J.; Lee, D.H.; Song, M.S. Subjective memory complaints and clinical characteristics in elderly Koreans: A questionnaire survey. Int. J. Nurs. Stud. 2007, 44, 1400–1405. [Google Scholar] [CrossRef]
- Shim, H.; Kim, M.; Won, C.W. Motoric cognitive risk syndrome is associated with processing speed and executive function, but not delayed free recall memory: The Korean frailty and aging cohort study (KFACS). Arch. Gerontol. Geriatr. 2020, 87, 103990. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.W.; Kim, M.H.; Kim, M.D.; Kim, B.J.; Kim, S.K.; Kim, J.L.; Moon, S.W.; Bae, J.N.; Woo, J.I.; et al. A nationwide survey on the prevalence and risk factors of late life depression in South Korea. J. Affect. Disord 2012, 138, 34–40. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.J.; Lee, S.B.; Huh, Y.; Choi, E.A.; Youn, J.C.; Jhoo, J.H.; Kim, J.S.; Woo, J.I.; Kim, K.W. Prevalence of major depressive disorder and minor depressive disorder in an elderly Korean population: Results from the Korean Longitudinal Study on Health and Aging (KLoSHA). J. Affect. Dis. 2010, 125, 234–240. [Google Scholar] [CrossRef]
- Callisaya, M.L.; Ayers, E.; Barzilai, N.; Ferrucci, L.; Guralnik, J.M.; Lipton, R.B.; Otahal, P.; Srikanth, V.K.; Verghese, J. Motoric Cognitive Risk Syndrome and Falls Risk: A Multi-Center Study. J. Alzheimers Dis. 2016, 53, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Beauchet, O.; Sekhon, H.; Schott, A.M.; Rolland, Y.; Muir-Hunter, S.; Markle-Reid, M.; Gagne, H.; Allali, G. Motoric Cognitive Risk Syndrome and Risk for Falls, Their Recurrence, and Postfall Fractures: Results From a Prospective Observational Population-Based Cohort Study. J. Am. Med. Dir. Assoc. 2019, 20, 1268–1273. [Google Scholar] [CrossRef]
- Lord, S.; Moyes, S.; Teh, R.; Port, W.; Muru-Lanning, M.; Bacon, C.J.; Wilkinson, T.; Kerse, N. Gait, cognition and falls over 5 years, and motoric cognitive risk in New Zealand octogenarians: Te Puawaitanga o Nga Tapuwae Kia Ora Tonu, LiLACS NZ. BMC Geriatr. 2020, 20, 43. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Tian, Q.; Carlson, M.C.; Xue, Q.L.; Ferrucci, L. Motoric cognitive risk syndrome: Integration of two early harbingers of dementia in older adults. Ageing Res. Rev. 2020, 58, 101022. [Google Scholar] [CrossRef]
- Won, C.W.; Lee, S.; Kim, J.; Chon, D.; Kim, S.; Kim, C.O.; Kim, M.K.; Cho, B.; Choi, K.M.; Roh, E.; et al. Korean frailty and aging cohort study (KFACS): Cohort profile. BMJ Open 2020, 10, e035573. [Google Scholar] [CrossRef] [Green Version]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Bae, J.N.; Cho, M.J. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J. Psychosom. Res. 2004, 57, 297–305. [Google Scholar] [CrossRef]
- Doi, T.; Verghese, J.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T. Motoric Cognitive Risk Syndrome: Prevalence and Risk Factors in Japanese Seniors. J. Am. Med. Dir. Assoc. 2015, 16, 1103.e21–1103.e25. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean Version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K): Clinical and Neuropsychological Assessment Batteries. J. Gerontol. Ser. B 2002, 57, P47–P53. [Google Scholar] [CrossRef] [Green Version]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Eng. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Close, J.; Ellis, M.; Hooper, R.; Glucksman, E.; Jackson, S.; Swift, C. Prevention of falls in the elderly trial (PROFET): A randomised controlled trial. Lancet 1999, 353, 93–97. [Google Scholar] [CrossRef]
- Uemura, K.; Shimada, H.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Lee, S.; Umegaki, H.; Kuzuya, M.; Suzuki, T. Effects of Mild Cognitive Impairment on the Development of Fear of Falling in Older Adults: A Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2015, 16, 1104.e9–1104.e13. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.E.; Myers, A.M. The activities-specific balance confidence (ABC) scale. J. Gerontol. Ser. A Biolog. Sci. Med. Sci. 1995, 50, M28–M34. [Google Scholar] [CrossRef] [PubMed]
- Moiz, J.A.; Bansal, V.; Noohu, M.M.; Gaur, S.N.; Hussain, M.E.; Anwer, S.; Alghadir, A. Activities-specific balance confidence scale for predicting future falls in Indian older adults. Clin. Interv. Aging 2017, 12, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.; Kim, S.; Won, C.; Choi, H.; Kim, B.; Park, M. Physical frailty predicts medical expenses in community-dwelling, elderly patients: Three-year prospective findings from living profiles of older people surveys in Korea. Eur. Geriatr. Med. 2015, 6, 412–416. [Google Scholar] [CrossRef]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S.; et al. Global prevalence of vision impairment and blindness: Magnitude and temporal trends, 1990-2010. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef]
- Yoo, M.; Kim, S.; Kim, B.S.; Yoo, J.; Lee, S.; Jang, H.C.; Cho, B.L.; Son, S.J.; Lee, J.H.; Park, Y.S.; et al. Moderate hearing loss is related with social frailty in a community-dwelling older adults: The Korean Frailty and Aging Cohort Study (KFACS). Arch. Gerontol. Geriatr. 2019, 83, 126–130. [Google Scholar] [CrossRef]
- Won, C.W. Korea Activities of Daily Living Scale and Korea Instrumental Activities of Daily Living Scale. J. Korean Geriatr. Soc. 2002, 6, 1–10. [Google Scholar]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Lee, K.U.; Lee, J.H.; Kim, K.W.; Jhoo, J.H.; Youn, J.C.; Kim, S.Y.; Woo, S.I.; Woo, J.I. A normative study of the mini-mental state examination in the Korean elderly. J. Korean Neuropsychiatr. Assoc. 2002, 41, 508. [Google Scholar]
- Lee, S.C.; Kim, W.H.; Chang, S.M.; Kim, B.S.; Lee, D.W.; Bae, J.N.; Cho, M.J. The use of the Korean version of Short Form Geriatric Depression Scale (SGDS-K) in the community dwelling elderly in Korea. J. Korean Geriatr. Psychiatry 2013, 17, 37. [Google Scholar]
- Jo, M.-W.; Yun, S.-C.; Lee, S.-I. Estimating quality weights for EQ-5D health states with the time trade-off method in South Korea. Value Health 2008, 11, 1186–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [PubMed] [Green Version]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Jang, Y.; Small, B.J.; Haley, W.E. Cross-cultural comparability of the Geriatric Depression Scale: Comparison between older Koreans and older Americans. Aging Ment. Health 2001, 5, 31–37. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, K.W.; Kim, T.H.; Park, J.H.; Lee, S.B.; Park, J.W.; McQuoid, D.R.; Steffens, D.C. Cross-cultural considerations in administering the center for epidemiologic studies depression scale. Gerontology 2011, 57, 455–461. [Google Scholar] [CrossRef]
- Chin, J.; Oh, K.J.; Seo, S.W.; Na, D.L. Are depressive symptomatology and self-focused attention associated with subjective memory impairment in older adults? Int. Psychogeriatr. 2014, 26, 573–580. [Google Scholar] [CrossRef]
- Schweizer, S.; Kievit, R.A.; Emery, T.; Cam, C.A.N.; Henson, R.N. Symptoms of depression in a large healthy population cohort are related to subjective memory complaints and memory performance in negative contexts. Psychol. Med. 2018, 48, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Slavin, M.J.; Brodaty, H.; Kochan, N.A.; Crawford, J.D.; Trollor, J.N.; Draper, B.; Sachdev, P.S. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am. J. Geriatr. Psychiatry 2010, 18, 701–710. [Google Scholar] [CrossRef]
- Loewenstein, D.A.; Barker, W.W.; Harwood, D.G.; Luis, C.; Acevedo, A.; Rodriguez, I.; Duara, R. Utility of a modified mini-mental state examination with extended delayed recall in screening for mild cognitive impairment and dementia among community dwelling elders. Int. J. Geriatr. Psychiatry 2000, 15, 434–440. [Google Scholar] [CrossRef]
- Fage, B.A.; Chan, C.C.; Gill, S.S.; Noel-Storr, A.H.; Herrmann, N.; Smailagic, N.; Nikolaou, V.; Seitz, D.P. Mini-Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a community setting. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Montejo Carrasco, P.; Montenegro-Pena, M.; Lopez-Higes, R.; Estrada, E.; Prada Crespo, D.; Montejo Rubio, C.; Garcia Azorin, D. Subjective Memory Complaints in healthy older adults: Fewer complaints associated with depression and perceived health, more complaints also associated with lower memory performance. Arch. Gerontol. Geriatr. 2017, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Payette, M.C.; Belanger, C.; Leveille, V.; Grenier, S. Fall-Related Psychological Concerns and Anxiety among Community-Dwelling Older Adults: Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152848. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Overall | Normal (without Impaired 3IR and Slow Gait) | Impaired 3IR only | Slow Gait only | MCR Using 3IR | p Value |
|---|---|---|---|---|---|---|
| (n = 2133) | (n = 854) | (n = 1081) | (n = 93) | (n = 105) | ||
| Sociodemographic factors | ||||||
| Age (years) | 75.6 ± 3.8 | 75.4 ± 3.9 | 75.7 ± 3.8 | 75.2 ± 3.8 | 75.8 ± 4.0 | 0.350 |
| Female sex | 1032 (48.4) | 468 (54.8) * | 476 (44.0) * | 48 (51.6) | 40 (38.1) | <0.001 |
| Education (years) | 9.7 ± 4.7 | 9.6 ± 4.7 b | 10.1 ± 4.5 d | 7.7 ± 5.3 b,d | 9.0 ± 4.6 | <0.001 |
| Residence | ||||||
| Urban | 652 (30.6) | 287 (33.6) | 311 (28.8) | 25 (26.9) | 29 (27.6) | 0.008 |
| Suburban | 976 (45.8) | 374 (43.8) | 523 (48.4) | 34 (36.6) | 45 (42.9) | |
| Rural | 505 (23.7) | 193 (22.6) | 247 (22.8) * | 34 (36.6) | 31 (29.5) | |
| Living alone | 423 (19.8) | 178 (20.8) | 193 (17.9) | 26 (28.0) | 26 (24.8) | 0.034 |
| Marital status (without partner) | 614 (28.8) | 259 (30.3) | 283 (26.2) | 37 (39.8) | 35 (33.3) | 0.011 |
| Basic livelihood security and/or medical care aid recipient | 149 (7.0) | 61 (7.1) | 61 (5.6) | 16 (17.2) * | 11 (10.5) | <0.001 |
| Lifestyle-related factors | ||||||
| Current smoker | 121 (5.7) | 43 (5.0) | 65 (6.0) | 5 (5.4) | 8 (7.6) | 0.650 |
| Alcohol consumption(≥2 to 3 times/week) | 405 (19.0) | 150 (17.6) | 222 (20.5) | 13 (14.0) | 20 (19.0) | 0.228 |
| Low physical activity | 169 (7.9) | 54 (6.3) * | 75 (6.9) | 20 (21.5) * | 20 (19.0) * | <0.001 |
| General health and medical conditions | ||||||
| BMI (kg/m2) | 24.5 ± 3.0 | 24.6 ± 3.0 | 24.4 ± 2.9 d | 25.1 ± 3.0 d | 24.8 ± 3.6 | 0.061 |
| <18.5 | 34 (1.6) | 10 (1.2) | 21 (1.9) | 0 (0.0) | 3 (2.9) * | 0.257 |
| 18.5–24.9 | 1213 (56.9) | 479 (56.1) | 630 (58.3) | 48 (51.6) | 56 (53.3) | |
| ≥25 | 886 (41.5) | 395 (42.7) | 430 (39.8) | 45 (48.4) | 46 (43.8) | |
| Number of drugs taken daily | 3.4 ± 2.9 | 3.3 ± 2.8 b,c | 3.3 ± 2.9 d,e | 4.7 ± 3.8 b,d | 4.2 ± 3.0 c,e | <0.001 |
| Number of diseases | 1.7 ± 1.2 | 1.7 ± 1.2 c | 1.6 ± 1.2 e | 1.9 ± 1.2 | 2.0 ± 1.3 c,e | 0.004 |
| Hypertension | 1211 (56.8) | 476 (55.7) | 611 (56.5) | 55 (59.1) | 69 (65.7) | 0.258 |
| Diabetes | 458 (21.5) | 160 (18.7) | 236 (21.8) | 23 (24.7) | 39 (37.1) * | <0.001 |
| Dyslipidemia | 718 (33.7) | 304 (35.6) | 353 (32.7) | 27 (29.0) | 34 (32.4) | 0.408 |
| Urinary incontinence | 65 (3.0) | 29 (3.4) | 29 (2.7) | 5 (5.4) | 2 (1.9) | 0.390 |
| Visual impairment | 39 (1.8) | 14 (1.6) | 18 (1.7) | 4 (4.3) | 3 (2.9) | 0.230 |
| Hearing impairment | 325 (15.2) | 126 (14.8) | 166 (15.4) | 15 (16.1) | 18 (17.1) | 0.915 |
| Poor nutritional status (MNA screening score ≤ 11) | 144 (6.8) | 56 (6.6) | 69 (6.4) | 11 (11.8) | 8 (7.6) | 0.239 |
| IADL disability | 246 (11.5) | 109 (12.8) | 107 (9.9) * | 19 (20.4) * | 11 (10.5) * | 0.010 |
| Psychological factors | ||||||
| General cognitive function (MMSE score) | 27.0 ± 1.7 | 27.8 ± 1.7 a,c | 26.4 ± 1.4 a,d | 27.6 ± 2.0 d,f | 26.0 ± 1.4 c,f | <0.001 |
| Fair/poor self-perceived health | 533 (25.0) | 201 (23.5) | 244 (22.6) | 45 (48.4) * | 43 (41.0) * | <0.001 |
| Depressive symptoms (GDS score ≥ 6) | 383 (18.0) | 141 (16.5) | 179 (16.6) | 31 (33.3) * | 32 (30.5) * | <0.001 |
| Quality of life (EQ-5D score) | 0.899 ± 0.117 | 0.903 ± 0.113 b,c | 0.909 ± 0.111 d,e | 0.824 ± 0.155 b,d | 0.830 ± 0.132 c,e | 0.007 |
| Physical functions | ||||||
| Handgrip strength (kg) | 27.4 ± 7.5 | 27.0 ± 7.4 a | 27.9 ± 7.6 a | 25.9 ± 7.6 | 26.7 ± 6.9 | <0.001 |
| Usual walking speed (m/s) | 1.14 ± 0.24 | 1.19 ± 0.21 b,c | 1.17 ± 0.22 d,e | 0.78 ± 0.13 b,d | 0.78 ± 0.13 c,e | <0.001 |
| Timed get up and go test (s) † | 10.0 ± 2.2 | 9.6 ± 2.0 b,c | 9.7 ± 1.8 d,e | 12.5 ± 3.4 b,d | 12.5 ± 3.1 c,e | <0.001 |
| SPPB score † | 11.1 ± 1.3 | 11.2 ± 1.1 b,c | 11.2 ± 1.1 d,e | 10.1 ± 2.0 b,d | 9.9 ± 1.9 c,e | <0.001 |
| MCR syndrome Using three-item recall test | ||||||
| Impaired three-item recall | 1186 (55.6) | 0 (0) | 1081 (100) | 0 (0) | 105 (100) | <0.001 |
| Slow gait | 198 (9.3) | 0 (0) | 0 (0) | 93 (100) | 105 (100) | <0.001 |
| Dependent Variables | Odds Ratio (95% Confidence Interval) (p-Value) | ||||||
|---|---|---|---|---|---|---|---|
| Normal (without Impaired 3IR and Slow Gait) | Impaired 3IR only | p | Slow Gait only | p | MCR Using 3IR | p | |
| Experience of falls in the past 1 year | |||||||
| Model 1 | Ref. | 0.971 (0.754, 1.251) | 0.820 | 1.406 (0.823, 2.402) | 0.212 | 2.157 (1.282, 2.255) | 0.002 |
| Model 2 | 0.969 (0.753, 1.248) | 0.809 | 1.349 (0.784, 2.320) | 0.279 | 2.098 (1.288, 3.416) | 0.003 | |
| Model 3 | 0.972 (0.753, 1.254) | 0.827 | 1.257 (0.725, 2.179) | 0.414 | 2.080 (1.271, 3.404) | 0.004 | |
| Model 4 | 0.959 (0.742, 1.239) | 0.748 | 1.166 (0.667, 2.038) | 0.590 | 1.915 (1.160, 3.160) | 0.011 | |
| Recurrent falls (≥ twice) | |||||||
| Model 1 | Ref. | 0.789 (0.523, 1.189) | 0.257 | 1.881 (0.921, 3.840) | 0.083 | 2.745 (1.446, 5.213) | 0.002 |
| Model 2 | 0.785 (0.521, 1.184) | 0.248 | 1.686 (0.811, 3.505) | 0.162 | 2.503 (1.302, 4.811) | 0.006 | |
| Model 3 | 0.809 (0.535, 1.223) | 0.315 | 1.563 (0.745, 3.280) | 0.237 | 2.581 (1.329, 5.012) | 0.005 | |
| Model 4 | 0.778 (0.513, 1.180) | 0.237 | 1.361 (0.642, 2.889) | 0.422 | 2.194 (1.115, 4.318) | 0.023 | |
| Falls with injury | |||||||
| Model 1 | Ref. | 0.980 (0.766, 1.252) | 0.869 | 1.647 (0.992, 2.735) | 0.054 | 2.207 (1.380, 3.530) | 0.001 |
| Model 2 | 0.977 (0.764, 1.250) | 0.856 | 1.585 (0.949, 2.648) | 0.079 | 2.151 (1.340, 3.454) | 0.002 | |
| Model 3 | 0.980 (0.765, 1.255) | 0.871 | 1.493 (0.888, 2.511) | 0.131 | 2.141 (1.328, 3.452) | 0.002 | |
| Model 4 | 0.967 (0.754, 1.240) | 0.790 | 1.392 (0.821, 2.360) | 0.219 | 1.982 (1.220, 3.220) | 0.006 | |
| Falls with fracture | |||||||
| Model 1 | Ref. | 0.660 (0.373, 1.165) | 0.660 | 2.442 (0.996, 5.987) | 0.051 | 3.133 (1.404, 6.988) | 0.005 |
| Model 2 | 0.659 (0.373, 1.164) | 0.151 | 1.967 (0.780, 4.961) | 0.152 | 2.764 (1.224, 6.237) | 0.014 | |
| Model 3 | 0.662 (0.372, 1.180) | 0.162 | 1.727 (0.671, 4.444) | 0.257 | 2.722 (1.185, 6.251) | 0.018 | |
| Model 4 | 0.648 (0.363, 1.157) | 0.143 | 1.593 (0.608, 4.171) | 0.343 | 2.508 (1.086, 5.791) | 0.031 | |
| Fear of falling | |||||||
| Model 1 | Ref. | 0.969 (0.769, 1.221) | 0.789 | 3.090 (1.901, 5.023) | <0.001 | 3.851 (2.409, 6.157) | <0.001 |
| Model 2 | 0.967 (0.767, 1.218) | 0.776 | 2.885 (1.764, 4.720) | <0.001 | 3.664 (2.283, 5.878) | <0.001 | |
| Model 3 | 0.981 (0.776, 1.241) | 0.875 | 2.604 (1.575, 4.305) | <0.001 | 3.407 (2.111, 5.497) | <0.001 | |
| Model 4 | 0.954 (0.751, 1.212) | 0.700 | 2.218 (1.314, 3.746) | 0.003 | 3.000 (1.830, 4.917) | <0.001 | |
| Low activities-specific balance confidence | |||||||
| Model 1 | Ref. | 1.037 (0.713, 1.508) | 0.849 | 5.403 (2.960, 9.863) | <0.001 | 5.269 (2.881, 9.639) | <0.001 |
| Model 2 | 1.010 (0.692, 1.474) | 0.957 | 4.358 (2.335, 8.135) | <0.001 | 4.609 (2.477, 8.576) | <0.001 | |
| Model 3 | 1.087 (0.733, 1.613) | 0.678 | 4.094 (2.087, 8.032) | <0.001 | 4.320 (2.268, 8.230) | <0.001 | |
| Model 4 | 0.978 (0.648, 1.478) | 0.917 | 2.994 (1.467, 6.108) | 0.003 | 3.134 (1.571, 6.253) | 0.001 | |
| Dependent Variables | Odds Ratio (95% Confidence Interval) (p-Value) | ||||||
|---|---|---|---|---|---|---|---|
| Normal (without SCCs and Slow Gait) | SCCs only | p | Slow Gait only | p | MCR Using SCCs | p | |
| Experience of falls in the past 1 year | |||||||
| Model 1 | Ref. | 0.705 (0.519, 0.959) | 0.026 | 2.047 (1.133, 3.699) | 0.018 | 1.080 (0.647, 1.801) | 0.769 |
| Model 2 | 0.711 (0.523, 0.967) | 0.029 | 1.989 (1.095, 3.612) | 0.024 | 1.061 (0.634, 1.774) | 0.823 | |
| Model 3 | 0.770 (0.562, 1.055) | 0.103 | 1.955 (1.066, 3.586) | 0.030 | 1.123 (0.667, 1.888) | 0.663 | |
| Model 4 | 0.902 (0.650, 1.253) | 0.540 | 1.865 (1.008, 3.452) | 0.047 | 1.254 (0.740, 2.126) | 0.400 | |
| Recurrent falls (≥ twice) | |||||||
| Model 1 | Ref. | 0.531 (0.336, 0.840) | 0.007 | 2.631 (1.255, 5.517) | 0.010 | 1.165 (0.574, 2.367) | 0.672 |
| Model 2 | 0.550 (0.346, 0.872) | 0.011 | 2.430 (1.145, 5.156) | 0.021 | 1.106 (0.539, 2.271) | 0.784 | |
| Model 3 | 0.609 (0.378, 0.979) | 0.041 | 2.411(1.113, 5.220) | 0.026 | 1.214 (0.586, 2.513) | 0.601 | |
| Model 4 | 0.790 (0.479, 1.304) | 0.356 | 2.269 (1.038, 4.958) | 0.040 | 1.410 (0.672, 2.960) | 0.364 | |
| Falls with injury | |||||||
| Model 1 | Ref. | 0.709 (0.526, 0.955) | 0.024 | 2.021 (1.127, 3.622) | 0.018 | 1.258 (0.776, 2.040) | 0.352 |
| Model 2 | 0.716 (0.531, 0.965) | 0.028 | 1.961 (1.089, 3.533) | 0.025 | 1.241 (0.763, 2.047) | 0.384 | |
| Model 3 | 0.766 (0.565, 1.040) | 0.088 | 1.950 (1.073, 3.541) | 0.028 | 1.299 (0.794, 2.123) | 0.297 | |
| Model 4 | 0.897 (0.652, 1.235) | 0.505 | 1.864 (1.018, 3.416) | 0.044 | 1.454 (0.882, 2.396) | 0.142 | |
| Falls with fracture | |||||||
| Model 1 | Ref. | 0.969 (0.473, 1.983) | 0.931 | 7.533 (2.893, 19.614) | <0.001 | 1.682 (0.580, 4.874) | 0.338 |
| Model 2 | 0.998 (0.485, 2.051) | 0.995 | 6.678 (2.528, 17.638) | <0.001 | 1.449 (0.493, 4.254) | 0.500 | |
| Model 3 | 1.193 (0.561, 2.534) | 0.647 | 8.001 (2.898, 22.094) | <0.001 | 1.479 (0.491, 4.458) | 0.487 | |
| Model 4 | 1.491 (0.679, 3.273) | 0.319 | 7.738 (2.766, 21.651) | <0.001 | 1.763 (0.575, 5.410) | 0.321 | |
| Fear of falling | |||||||
| Model 1 | Ref. | 0.566 (0.428, 0.747) | <0.001 | 4.245 (2.223, 8.103) | <0.001 | 1.712 (1.088, 2.696) | 0.020 |
| Model 2 | 0.569 (0.431, 0.752) | <0.001 | 4.047 (2.107, 7.773) | <0.001 | 1.632 (1.033, 2.580) | 0.036 | |
| Model 3 | 0.644 (0.484, 0.857) | 0.003 | 3.756 (1.945, 7.252) | <0.001 | 1.701 (1.068, 2.709) | 0.025 | |
| Model 4 | 0.874 (0.644, 1.185) | 0.385 | 3.719(1.861, 7.432) | <0.001 | 2.040 (1.260, 3.301) | 0.004 | |
| Low activities-specific balance confidence | |||||||
| Model 1 | Ref. | 0.414 (0.276, 0.621) | <0.001 | 3.951 (2.019, 7.733) | <0.001 | 2.198 (1.211, 3.990) | 0.010 |
| Model 2 | 0.434 (0.288, 0.654) | <0.001 | 3.449 (1.727, 6.890) | <0.001 | 1.952 (1.056, 3.610) | 0.033 | |
| Model 3 | 0.561 (0.362, 0.870) | 0.010 | 3.520 (1.683, 7.362) | 0.001 | 2.215 (1.153, 4.254) | 0.017 | |
| Model 4 | 0.829 (0.517, 1.331) | 0.437 | 2.722 (1.235, 6.001) | 0.013 | 2.748 (1.374, 5.495) | 0.004 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.; Kim, M.; Won, C.W. Motoric Cognitive Risk Syndrome Using Three-Item Recall Test and Its Associations with Fall-Related Outcomes: The Korean Frailty and Aging Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 3364. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103364
Shim H, Kim M, Won CW. Motoric Cognitive Risk Syndrome Using Three-Item Recall Test and Its Associations with Fall-Related Outcomes: The Korean Frailty and Aging Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(10):3364. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103364
Chicago/Turabian StyleShim, Hayoung, Miji Kim, and Chang Won Won. 2020. "Motoric Cognitive Risk Syndrome Using Three-Item Recall Test and Its Associations with Fall-Related Outcomes: The Korean Frailty and Aging Cohort Study" International Journal of Environmental Research and Public Health 17, no. 10: 3364. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103364





