Assessment of Risk Hospitalization due to Acute Respiratory Incidents Related to Ozone Exposure in Silesian Voivodeship (Poland)
Abstract
:1. Introduction
2. Material and Methods
2.1. Health Data
2.2. Environmental Data
2.3. Data Analysis
2.4. The Applied Models
2.4.1. The Almon Distributed Lag Model
2.4.2. The Poisson Distributed Lag Model
2.4.3. Distributed Lag Non-linear Models—DLNM
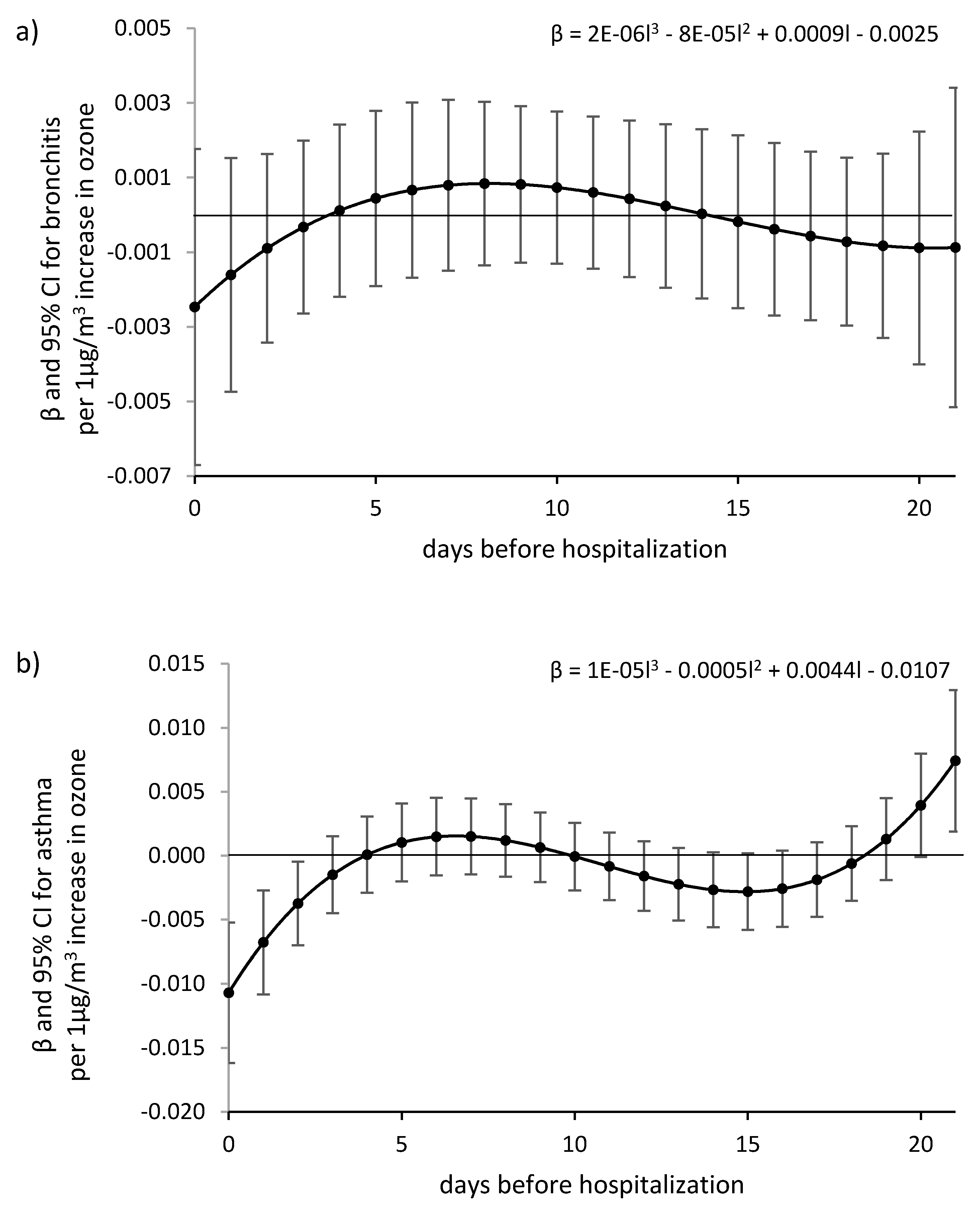
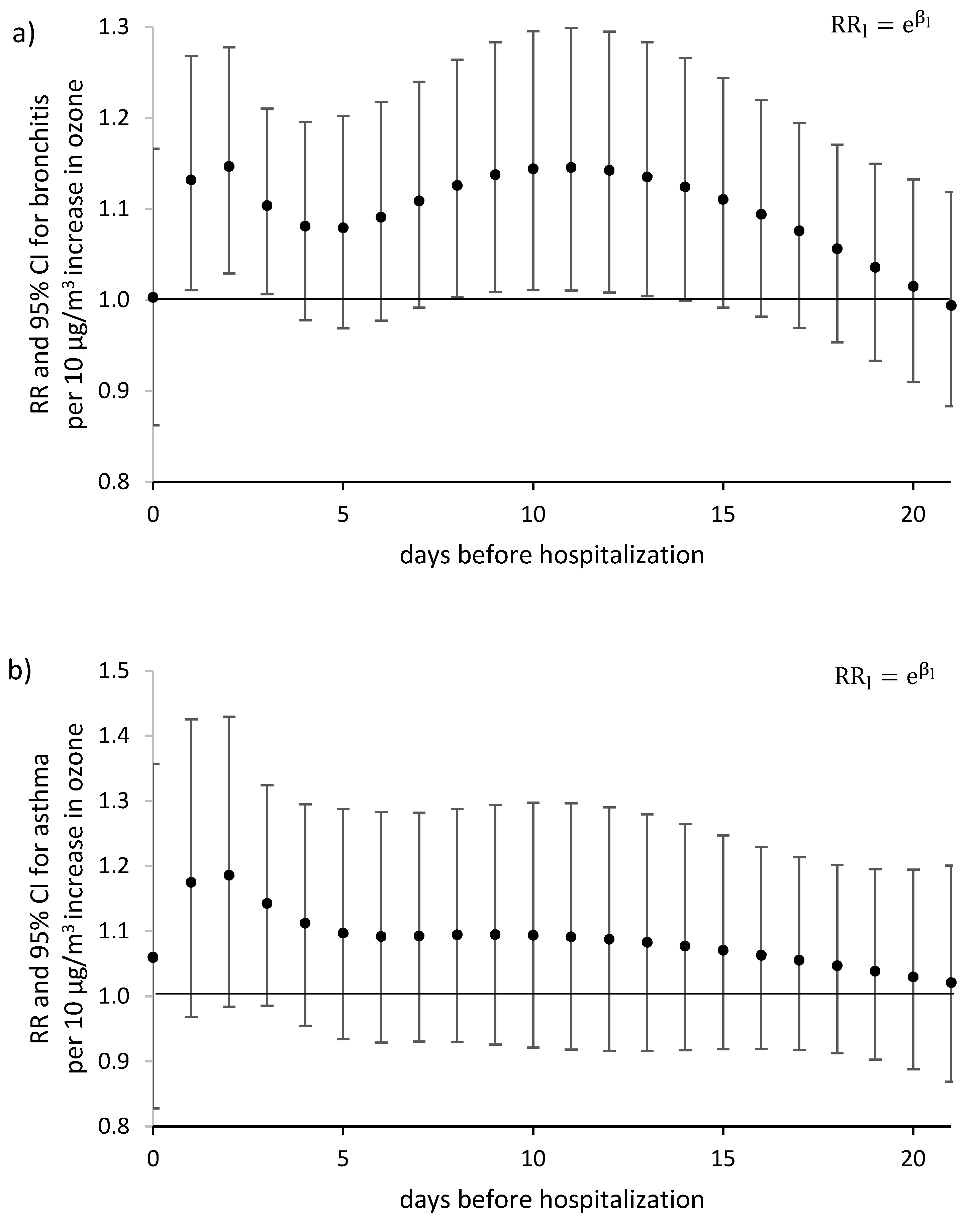
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chief Inspectorate for Environmental Protection. The State Environmental Monitoring system. Available online: http://www.gios.gov.pl/pl/stan-srodowiska/monitoring-jakosci-powietrza (accessed on 2 April 2020).
- The Airly Map: Quality Condition and Level of Air Pollution in Poland. Available online: https://airly.eu/map/pl (accessed on 2 April 2020).
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J.; Niewiadomska, E.; Czech, E. The Relationship between Daily Concentration of Fine Particulate Matter in Ambient Air and Exacerbation of Respiratory Diseases in Silesian Agglomeration, Poland. Int. J. Environ. Res. Public Heal. 2019, 16, 1131. [Google Scholar] [CrossRef] [Green Version]
- Wiech, A.K.; Marciniewicz-Mykieta, M. The State of the Environment in Poland. Sygnały2016; Chief Inspectorate for Environmental Protection: Warszawa, Poland, 2017. [Google Scholar]
- Office of the European Union. Air Quality in Europe – 2019 Report. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2019 (accessed on 2 April 2020).
- WHO Europe. Health Risks of Ozone from Long-Range Transboundary Air Pollution. WHO Regional Office for Europe. Copenhagen. 2008. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/78647/E91843.pdf?ua=1 (accessed on 2 April 2020).
- Malig, B.; Pearson, D.L.; Chang, Y.B.; Broadwin, R.; Basu, R.; Green, R.S.; Ostro, B. A Time-Stratified Case-Crossover Study of Ambient Ozone Exposure and Emergency Department Visits for Specific Respiratory Diagnoses in California (2005–2008). Environ. Heal. Perspect. 2016, 124, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.-Y.; Ding, H.; Jiang, L.-N.; Chen, S.-W.; Zheng, J.-P.; Qiu, M.; Zhou, Y.-X.; Chen, Q.; Guan, W.-J. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLOS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef] [PubMed]
- Plan of Adaptation to Climate Change in Cities over 100,000. Available online: http://klimada.mos.gov.pl/zmiany-klimatu-w-polsce/tendencje-zmian-klimatu/ (accessed on 2 April 2020).
- Air Quality Monitoring System. State Inspectorate of Environmental Protection in Katowice. Elevated Ozone Concentrations in the Air. Available online: http://powietrze.gios.gov.pl/pjp/content/show/1000605 (accessed on 2 April 2020).
- Giles, D. Explaining the Almon Distributed Lag Model. R-bloggers. 2017. Available online: https://www.r-bloggers.com/explaining-the-almon-distributed-lag-model/ (accessed on 2 April 2020).
- Stanisz, A. Logistic Regression Models. Applications in Medicine, Natural and Social Sciences; StatSoft: Kraków, Poland, 2016. (In Polish) [Google Scholar]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Central Statistical Office. Local Data Bank. Available online: https://bdl.stat.gov.pl/BDL/dane/podgrup/tablica (accessed on 2 April 2020).
- Air Quality Monitoring System. State Inspectorate of Environmental Protection in Katowice. 2017. Available online: http://powietrze.katowice.wios.gov.pl/dane-pomiarowe/automatyczne (accessed on 2 April 2020).
- Hornik, K. The Comprehensive R Archive Network. Wiley Interdiscip. Rev. Comput. Stat. 2012, 4, 394–398. [Google Scholar] [CrossRef]
- The Comprehensive R Archive Network. Stats package. Available online: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html (accessed on 2 April 2020).
- The Comprehensive R Archive Network. Dlnm package. Available online: https://cran.r-project.org/web/packages/dlnm/ (accessed on 2 April 2020).
- Gasparrini, A. Modelling Lagged Associations in Environmental Time Series Data. Epidemiology 2016, 27, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparrini, A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med. 2013, 33, 881–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation of the Minister of the Environment of 24 August 2012 on the Levels of Certain Substances in the Air. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20120001031 (accessed on 2 April 2020).
- Schwartz, J. The Distributed Lag between Air Pollution and Daily Deaths. Epidemiology 2000, 11, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Slama, A.; Śliwczyński, A.; Woźnica, J.; Zdrolik, M.; Wiśnicki, B.; Kubajek, J.; Turżańska-Wieczorek, O.; Gozdowski, D.; Wierzba, W.; Franek, E. Impact of air pollution on hospital admissions with a focus on respiratory diseases: a time-series multi-city analysis. Environ. Sci. Pollut. Res. 2019, 26, 16998–17009. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, K.J.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef]
- Armstrong, B. Models for the Relationship Between Ambient Temperature and Daily Mortality. Epidemiology 2006, 17, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Gasparrini, A. The Excess Winter Deaths Measure. Epidemiology 2016, 27, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicedo-Cabrera, A.M.; Sera, F.; Gasparrini, A. Hands-on Tutorial on a Modeling Framework for Projections of Climate Change Impacts on Health. Epidemiology 2019, 30, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Stieb, D.M.; Lavigne, É.; Chen, L.; Pinault, L.; Gasparrini, A.; Tjepkema, M. Air pollution in the week prior to delivery and preterm birth in 24 Canadian cities: a time to event analysis. Environ. Heal. 2019, 18, 1. [Google Scholar] [CrossRef]
- Lavigne, É.; Gasparrini, A.; Stieb, D.M.; Chen, H.; Yasseen, A.S.; Crighton, E.; To, T.; Weichenthal, S.; Villeneuve, P.J.; Cakmak, S.; et al. Maternal Exposure to Aeroallergens and the Risk of Early Delivery. Epidemiology 2017, 28, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Imai, C.; Hashizume, M. A Systematic Review of Methodology: Time Series Regression Analysis for Environmental Factors and Infectious Diseases. Trop. Med. Heal. 2015, 43, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, L.A.; Finch, S.J.; Lipfert, F.W.; Yu, Q. Comparing estimates of the effects of air pollution on human mortality obtained using different regression methodologies. Risk Anal. 1997, 17, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Burnett, R.T.; Beveridge, R.C.; Brook, J.R. Association between ozone and asthma emergency department visits in Saint John, New Brunswick, Canada. Environ. Heal. Perspect. 1996, 104, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, N.A.; Metcalf, W.J.; Ryu, S.Y.; Rutledge, A.; Coppes, M.J.; Grzymski, J.J.; Strickland, M.J.; Darrow, L.A. Acute associations between PM2.5 and ozone concentrations and asthma exacerbations among patients with and without allergic comorbidities. J. Expo. Sci. Environ. Epidemiol. 2020, 1–10. [Google Scholar] [CrossRef]
- Katsouyanni, K.; Samet, J.M.; Anderson, H.R.; Atkinson, R.; Le Tertre, A.; Medina, S.; Samoli, E.; Touloumi, G.; Burnett, R.T.; Krewski, D.; et al. Air pollution and health: a European and North American approach (APHENA). Res. Rep. (Health Eff. Institute) 2009, 142, 5–90. [Google Scholar]
- Peng, R.D.; Samoli, E.; Pham, L.; Dominici, F.; Touloumi, G.; Ramsay, T.; Burnett, R.T.; Krewski, D.; Le Tertre, A.; Cohen, A.; et al. Acute effects of ambient ozone on mortality in Europe and North America: results from the APHENA study. Air Qual. Atmos. Heal. 2012, 6, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, M.S.; Powell, K.E.; Hutwagner, L.; Graham, L.M.; Teague, W.G. Impact of changes in transportation and commuting behaviors during the 1996 Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA 2001, 285, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Lipsett, M.; Mann, J.; Braxton-Owens, H.; White, M.C. Air Pollution and Exacerbation of Asthma in African-American Children in Los Angeles. Epidemiology 2001, 12, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; Neas, L.; Dockery, D.W.; Redline, S.; Tager, I. The effect of air pollution on inner-city children with asthma. Eur. Respir. J. 2002, 19, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.B.; Holguin, F.C.; Ryan, P.B.; Mannino, D.; Erzurum, S.C.; Teague, W.G. Association of Ambient Ozone Exposure with Airway Inflammation and Allergy in Adults with Asthma. J. Asthma 2009, 46, 777–785. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Total | 2016 | 2017 | ||||
|---|---|---|---|---|---|---|---|
| June | July | August | June | July | August | ||
| Air Temperature (°C) | 9.2 (13.9) −18.4–26.8 | 17.5 (4) 14.4–26.2 | 19.2 (5) 13.6–25 | 17.4 (4.2) 11.6–23.2 | 18.4 (4) 14.4–26.2 | 17.6 (3.9) 11–25.4 | 19.6 (6.6) 14.4–25.4 |
| Relative Humidity (%) | 81.5 (18.6) 48–99 | 69 (16.8) 55.3–96.5 | 79.5 (21.5) 60.3–94.5 | 74.8 (10.3) 66.8–97 | 67.6 (10.5) 51.3–95 | 73.7 (13.7) 58–92.7 | 73.7 (13.7) 60.3–96.3 |
| Wind Speed (m/s) | 0.8 (0.8) 0–2.6 | 0.4 (0.8) 0–1 | 0.4 (0.8) 0–1.4 | 0.5 (0.8) 0–1.3 | 0.8 (0.8) 0–1.8 | 0.6 (0.6) 0–1.8 | 0.4 (0.8) 0–1.2 |
| O3 (μg/m3) | 47 (33.3) 3.7–99.3 | 63.8 (18.3) 36–89.7 | 57.3 (26) 36–89.7 | 52 (18) 28–65.7 | 71.3 (16) 44–99.3 | 58.7 (15.3) 38.7–98.7 | 66 (29) 35–94.3 |
| O3 8 h (μg/m3) | 71.3 (50.3) 4.5–150.3 | 99.8 (24) 55–149 | 92 (31.3) 52.7–129.3 | 83.7 (32) 44–115.7 | 103.3 (31.3) 74.7–150.3 | 94.7 (27.7) 56.7–144.3 | 101.7 (48.7) 60.7–146 |
| Number of Days with O3 8 h ≥ 120 μg/m3 | 44 | 5 | 4 | 0 | 9 | 5 | 12 |
| Number and Percentage of Hospitalizations | |||||||
| Bronchitis | 4674 (100) | 96 (2.1) | 67 (1.4) | 76 (1.6) | 88 (1.9) | 70 (1.5) | 66 (1.4) |
| Asthma | 3815 (100) | 153 (4) | 153 (4) | 165 (4.3) | 168 (4.4) | 156 (4.1) | 154 (4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niewiadomska, E.; Kowalska, M.; Niewiadomski, A.; Skrzypek, M.; Kowalski, M.A. Assessment of Risk Hospitalization due to Acute Respiratory Incidents Related to Ozone Exposure in Silesian Voivodeship (Poland). Int. J. Environ. Res. Public Health 2020, 17, 3591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103591
Niewiadomska E, Kowalska M, Niewiadomski A, Skrzypek M, Kowalski MA. Assessment of Risk Hospitalization due to Acute Respiratory Incidents Related to Ozone Exposure in Silesian Voivodeship (Poland). International Journal of Environmental Research and Public Health. 2020; 17(10):3591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103591
Chicago/Turabian StyleNiewiadomska, Ewa, Małgorzata Kowalska, Adam Niewiadomski, Michał Skrzypek, and Michał A. Kowalski. 2020. "Assessment of Risk Hospitalization due to Acute Respiratory Incidents Related to Ozone Exposure in Silesian Voivodeship (Poland)" International Journal of Environmental Research and Public Health 17, no. 10: 3591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103591





