Bisphenols and Oxidative Stress Biomarkers—Associations Found in Human Studies, Evaluation of Methods Used, and Strengths and Weaknesses of the Biomarkers
Abstract
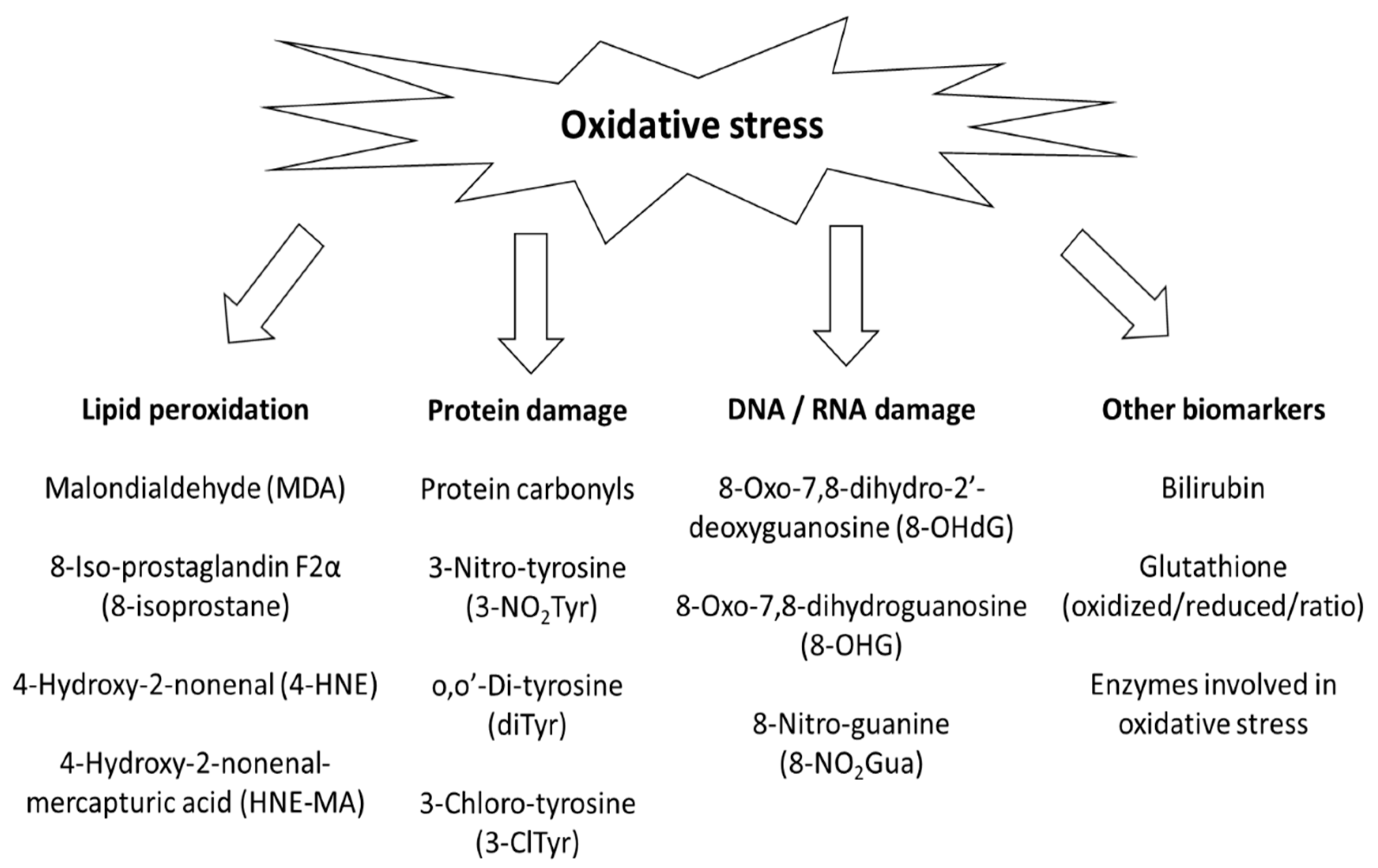
:1. Introduction
2. Materials and Methods
3. Results
3.1. Literature Search Results
3.2. Associations between Bisphenol Exposure and Oxidative Stress Biomarkers in Human Studies
3.2.1. Diseases and Adverse Health Conditions
3.2.2. Pregnant Women and Their Fetuses/Newborns
3.2.3. Children and Adolescents
3.2.4. Adults
3.2.5. Elderly
3.2.6. Exposures Based on Residence Area
3.3. Suggestions of Analytical Methods for the Identified Biomarkers of Oxidative Stress and Evaluation of Their Strengths and Weaknesses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Health Endpoint | Bisphenol AND (MeSH Terms OR synonym) |
|---|---|
| Behavior, neurodevelopment, and neurodegeneration | (Bisphenol) AND (((((((((((((“Behavior”[Mesh]) OR (“Behavior and Behavior Mechanisms”[Mesh] OR “Reproductive Behavior”[Mesh])) OR “Social Behavior Disorders”[Mesh]) OR (“Child Behavior Disorders”[Mesh] OR “Adolescent Behavior”[Mesh])) OR “Antisocial Personality Disorder”[Mesh]) OR (“Infant Behavior”[Mesh] OR “Spatial Behavior”[Mesh])) OR “Sucking Behavior”[Mesh]) OR (“Sexual Behavior, Animal”[Mesh] OR “Sexual Behavior”[Mesh])) OR (“Paternal Behavior”[Mesh] OR “Maternal Behavior”[Mesh] OR “Impulsive Behavior”[Mesh] OR “Feeding Behavior”[Mesh] OR “Exploratory Behavior”[Mesh])) OR (“Compulsive Behavior”[Mesh] OR “Child Behavior”[Mesh] OR “Behavior, Animal”[Mesh])) OR “Mental Disorders”[Mesh])) OR (Behavior OR Neurobehavior OR Neurodevelopment OR Neurology OR Parkinson OR Alzheimer OR Autism OR Hyperactivity OR ASD OR ADHD OR mental retardation OR IQ loss OR internalizing OR externalizing)) |
| Cancer | (Bisphenol) AND (((((((“Neoplasms”[Mesh] OR “Uterine Cervical Neoplasms”[Mesh] OR “Urologic Neoplasms”[Mesh] OR “Liver Neoplasms”[Mesh] OR “Hereditary Breast and Ovarian Cancer Syndrome”[Mesh] OR “Early Detection of Cancer”[Mesh]) OR (“Urogenital Neoplasms”[Mesh] OR “Testicular Neoplasms”[Mesh] OR “Endometrial Neoplasms”[Mesh] OR “Vaginal Neoplasms”[Mesh] OR “Uterine Neoplasms”[Mesh])) OR (“Prostatic Neoplasms”[Mesh] OR “Ovarian Neoplasms”[Mesh] OR “Endocrine Gland Neoplasms”[Mesh])) OR (“Breast Neoplasms”[Mesh] OR “Neoplasms, Germ Cell and Embryonal”[Mesh] OR “Tumor Microenvironment”[Mesh])) OR (“Thyroid Neoplasms”[Mesh] OR “Pituitary Neoplasms”[Mesh] OR “Brain Neoplasms”[Mesh]))) OR (Cancer OR hormone-dependent cancer OR neoplasm OR malignant tumor OR tumor OR tumour) OR (Colon neoplasms)) |
| Endocrine diseases | (Bisphenol) AND (“Endocrine System”[Mesh] OR “Endocrine Glands”[Mesh] OR “Endocrine System Diseases”[Mesh] OR “Hormones”[Mesh] OR “Gonadal Hormones”[Mesh] OR “Placental Hormones”[Mesh] OR “Pituitary Hormones”[Mesh] OR “Growth Hormone”[Mesh] OR “Thyroid Hormones”[Mesh] OR “Gastrointestinal Hormones”[Mesh] OR “Sex Hormone-Binding Globulin”[Mesh] OR “Adrenocorticotropic Hormone”[Mesh] OR “Adrenal Cortex Hormones”[Mesh] OR Endocrine system OR hypothyroidism OR hyperthyroidism OR adrenal) |
| Immune system and allergy | (bisphenol) AND (“Allergy and Immunology”[Mesh] OR “Hypersensitivity”[Mesh] OR “Rhinitis, Allergic, Seasonal”[Mesh] OR “Food Hypersensitivity”[Mesh] OR “Drug Hypersensitivity”[Mesh] OR “Shellfish Hypersensitivity”[Mesh] OR Allergy OR Hypersensitive OR respiratory allergy OR gastrointestinal allergy OR multiple chemical sensitivity OR allergic hypersensitivity disease OR contact allergy OR “Immune System”[Mesh] OR “Immune System Diseases”[Mesh] OR Immune system OR autoimmune disease OR cytokines OR white cells OR innate immune system OR adaptive immune system) |
| Obesity, metabolic disorders and cardiovascular diseases | (Bisphenol) AND (“Metabolic Syndrome”[Mesh] OR “Nutritional and Metabolic Diseases”[Mesh] OR “Metabolic Diseases”[Mesh] OR “Metabolism”[Mesh] OR “Glucose Metabolism Disorders”[Mesh] OR “Acidosis”[Mesh] OR “Metabolome”[Mesh] OR “Metabolomics”[Mesh] OR “Receptor, Insulin”[Mesh] OR “Lipolysis”[Mesh] OR “Gout”[Mesh] OR “Diabetes Mellitus, Type 2”[Mesh] OR “Acidosis, Renal Tubular”[Mesh] OR “Homocysteinemia” [Supplementary Concept] OR “Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha”[Mesh] OR “Obesity”[Mesh] OR “Pediatric Obesity”[Mesh] OR “Obesity, Abdominal”[Mesh] OR “Abdominal obesity metabolic syndrome” [Supplementary Concept] OR “Cardiovascular System”[Mesh] OR “Cardiovascular Abnormalities”[Mesh] OR “Pregnancy Complications, Cardiovascular”[Mesh] OR “Cardiovascular Diseases”[Mesh] OR “Myocardial Infarction”[Mesh] OR obesity OR abdominal obesity OR waist hip ratio OR adipose tissue OR adypokine OR visceral fat OR body fat OR overweight OR Metabolic OR Metabolic disorder OR Metabolic syndrome OR glucose homeostasis OR Hyperlipidemia OR Dyslipidemia OR hypertriglyceridemia OR HOMA-IR OR insulin resistance OR pancreas OR liver OR kidney) |
| Reproductive diseases | (Bisphenol) AND (reproductive OR puberty OR pregnancy OR infertility OR semen quality OR placenta OR anogenital distance OR hypospadia OR cryptorchidism OR “Reproductive Health”[Mesh] OR “Reproductive Medicine”[Mesh] OR “Reproduction”[Mesh] OR “Reproductive Techniques, Assisted”[Mesh] OR “Infertility”[Mesh]) |
Appendix B
Appendix B.1. Adverse Outcome Pathways (AOPs) and Key Events (KEs) Involving BPA or the Biomarkers of Oxidative Stress Reported in the Human Studies (Accessed 15 April 2020)
References
- Mukmeneva, N.; Gotlib, E.; Verizhnikov, M.; Nagumanova, G.; Grinberg, L.; Chakirov, R. The influence of bisphenol antioxidants on thermo-oxidative degradation of plasticised poly(vinyl chloride). Polym. Degrad. Stabil. 2001, 73, 383–386. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs: Executive Summary. EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). 2015. Available online: https://0-efsa-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.2903/j.efsa.2015.3978 (accessed on 20 May 2020).
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, S.; Uppu, S.; Claville, M.O.; Uppu, R.M. Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: Implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods. 2013, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Roy, D. In vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s). Biochem. Biophys. Res. Commun. 1995, 210, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Roy, D. In-Vivo DNA Adduct Formation by Bisphenol-A. Environ. Mol. Mutagen. 1995, 26, 60–66. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Hofer, T. Oxidation of 2‘-deoxyguanosine by H2O2-ascorbate: Evidence against free OH• and thermodynamic support for two-electron reduction of H2O2. J. Chem. Soc. Perk. Trans. 2 2001, 210–213. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Hofer, T.; Badouard, C.; Bajak, E.; Ravanat, J.L.; Mattsson, A.; Cotgreave, I.A. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 2005, 386, 333–337. [Google Scholar] [CrossRef]
- Alary, J.; Bravais, F.; Cravedi, J.P.; Debrauwer, L.; Rao, D.; Bories, G. Mercapturic acid conjugates as urinary end metabolites of the lipid peroxidation product 4-hydroxy-2-nonenal in the rat. Chem. Res. Toxicol. 1995, 8, 34–39. [Google Scholar] [CrossRef]
- Hu, M.L. Measurement of Protein Thiol-Groups and Glutathione in Plasma. Method. Enzymol. 1994, 233, 380–385. [Google Scholar]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodriguez-Carrillo, A.; Fini, J.-B.; Hofer, T.; Steffensen, I.-L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020. manuscript accepted. [Google Scholar]
- Dallio, M.; Masarone, M.; Errico, S.; Gravina, A.G.; Nicolucci, C.; Di Sarno, R.; Gionti, L.; Tuccillo, C.; Persico, M.; Stiuso, P.; et al. Role of bisphenol A as environmental factor in the promotion of non-alcoholic fatty liver disease: In vitro and clinical study. Aliment. Pharm. Ther. 2018, 47, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic Steatohepatitis Clinical Research, N., Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Kuang, H.; Fan, R.; Cha, C.; Li, G.; Luo, Z.; Pang, Q. Relationship between bisphenol A exposure and attention-deficit/hyperactivity disorder: A case-control study for primary school children in Guangzhou, China. Environ. Pollut. 2018, 235, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Metwally, F.M.; Rashad, H.; Zeidan, H.M.; Kilany, A.; Abdol Raouf, E.R. Study of the Effect of Bisphenol A on Oxidative Stress in Children with Autism Spectrum Disorders. Indian J. Clin. Biochem. 2018, 33, 196–201. [Google Scholar] [CrossRef]
- Kondolot, M.; Ozmert, E.N.; Asci, A.; Erkekoglu, P.; Oztop, D.B.; Gumus, H.; Kocer-Gumusel, B.; Yurdakok, K. Plasma phthalate and bisphenol a levels and oxidant-antioxidant status in autistic children. Environ. Toxicol. Phar. 2016, 43, 149–158. [Google Scholar] [CrossRef]
- Erden, E.S.; Motor, S.; Ustun, I.; Demirkose, M.; Yuksel, R.; Okur, R.; Oktar, S.; Yakar, Y.; Sungur, S.; Gokce, C. Investigation of Bisphenol A as an endocrine disruptor, total thiol, malondialdehyde, and C-reactive protein levels in chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3477–3483. [Google Scholar]
- Omran, G.A.; Gaber, H.D.; Mostafa, N.A.M.; Abdel-Gaber, R.M.; Salah, E.A. Potential hazards of bisphenol A exposure to semen quality and sperm DNA integrity among infertile men. Reprod. Toxicol. 2018, 81, 188–195. [Google Scholar] [CrossRef]
- Yang, M.; Lee, H.S.; Hwang, M.W.; Jin, M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: Single blind, randomized clinical trial of efficacy and safety. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod. Toxicol. 2016, 66, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Watkins, D.J.; Ferguson, K.K.; Del Toro, L.V.A.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Envir. Heal. 2015, 218, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga-Lopez, A.; Pennathur, S.; Kannan, K.; Patisaul, H.B.; Dolinoy, D.C.; Zeng, L.X.; Padmanabhan, V. Impact of Gestational Bisphenol A on Oxidative Stress and Free Fatty Acids: Human Association and Interspecies Animal Testing Studies. Endocrinology 2015, 156, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.F.; Wang, P.W.; Huang, L.W.; Lai, C.H.; Yang, W.N.; Wu, K.Y.; Lu, C.S.A.; Chen, H.C.; Chen, M.L. Prenatal Nonylphenol and Bisphenol A Exposures and Inflammation Are Determinants of Oxidative/Nitrative Stress: A Taiwanese Cohort Study. Environ. Sci. Technol. 2017, 51, 6422–6429. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Wang, P.W.; Huang, L.W.; Lin, M.H.; Yang, W.; Chen, H.C.; Yu, K.P.; Chen, M.L. Interactive effects of nonylphenol and bisphenol A exposure with oxidative stress on fetal reproductive indices. Environ. Res. 2018, 167, 567–574. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, Y.F.; Wang, P.W.; Lai, C.H.; Huang, L.W.; Chen, H.C.; Lin, M.H.; Yang, W.; Mao, I.F.; Chen, M.L. Associations between prenatal exposure to bisphenol a and neonatal outcomes in a Taiwanese cohort study: Mediated through oxidative stress? Chemosphere 2019, 226, 290–297. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Y.; Shao, Y.; Qu, W.; Chen, Y.; Jiang, Q. Urinary bisphenol analogues concentrations and biomarkers of oxidative DNA and RNA damage in Chinese school children in East China: A repeated measures study. Environ. Pollut. 2019, 254(Pt. A), 112921. [Google Scholar] [CrossRef]
- Rocha, B.A.; Asimakopoulos, A.G.; Honda, M.; da Costa, N.L.; Barbosa, R.M.; Barbosa, F., Jr.; Kannan, K. Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ. Int. 2018, 116, 269–277. [Google Scholar] [CrossRef]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P.; Bisphenol, A. Tobacco Smoke, and Age as Predictors of Oxidative Stress in Children and Adolescents. Int. J. Environ. Res. Public Health. 2019, 16, 2025. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.S.; Rui, C.Y.; Dai, Y.Y.; Pang, Q.H.; Li, Y.R.; Fan, R.F.; Lu, S.Y. Exposure of children to BPA through dust and the association of urinary BPA and triclosan with oxidative stress in Guangzhou, China. Environ. Sci.-Proc. Imp. 2016, 18, 1492–1499. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, C.; Shen, Y.; Wang, Q.; Pan, A.; Yang, P.; Chen, Y.J.; Deng, Y.L.; Lu, Q.; Cheng, L.M.; et al. Urinary levels of bisphenol A, F and S and markers of oxidative stress among healthy adult men: Variability and association analysis. Environ. Int. 2019, 123, 301–309. [Google Scholar] [CrossRef]
- Hong, Y.C.; Park, E.Y.; Park, M.S.; Ko, J.A.; Oh, S.Y.; Kim, H.; Lee, K.H.; Leem, J.H.; Ha, E.H. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett. 2009, 184, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Hong, Y.C.; Oh, S.Y.; Park, M.S.; Kim, H.; Leem, J.H.; Ha, E.H. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ. Res. 2009, 109, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, A.G.; Xue, J.C.; De Carvalho, B.P.; Iyer, A.; Abualnaja, K.O.; Yaghmoor, S.S.; Kumosani, T.A.; Kannan, K. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 2016, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, S.; Dong, T.; Han, Y.; Xue, J.; Wanjaya, E.R.; Fang, M. The occurrence of bisphenol plasticizers in paired dust and urine samples and its association with oxidative stress. Chemosphere 2019, 216, 472–478. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.J.; Jeon, J.; Jung, K.J.; Jee, S.H. Inverse associations of bisphenol A and phthalate metabolites with serum bilirubin levels in Korean population. Environ. Sci. Pollut. Res. Int. 2019, 26, 26685–26695. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Kasai, H.; Lee, H.S.; Kang, Y.; Park, J.Y.; Yang, M. Inhibition by wheat sprout (Triticum aestivum) juice of bisphenol A-induced oxidative stress in young women. Mutat. Res.-Gen. Tox. En. 2011, 724, 64–68. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.R.; Hong, Y.C. Modification of the association of bisphenol A with abnormal liver function by polymorphisms of oxidative stress-related genes. Environ. Res. 2016, 147, 324–330. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, Y.C. Increase of urinary malondialdehyde level by bisphenol A exposure: A longitudinal panel study. Environ. Health-Glob. 2017, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Xue, J.C.; Gao, C.Z.; Qiu, R.L.; Li, Y.X.; Li, X.; Huang, M.Z.; Kannan, K. Urinary Concentrations of Bisphenols and Their Association with Biomarkers of Oxidative Stress in People Living Near E-Waste Recycling Facilities in China. Environ. Sci. Technol. 2016, 50, 4045–4053. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and Thiobarbituric Acid-Reactivity as Diagnostic Indexes of Lipid-Peroxidation and Peroxidative Tissue-Injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Zelzer, S.; Oberreither, R.; Bernecker, C.; Stelzer, I.; Truschnig-Wilders, M.; Fauler, G. Measurement of total and free malondialdehyde by gas-chromatography mass spectrometry--comparison with high-performance liquid chromatography methology. Free Radic. Res. 2013, 47, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the Lipid Peroxidation Biomarkers and Methodological Aspects for Malondialdehyde Quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Suchy, M.T.; Trettin, A.; Modun, D.; Stuke, N.; Maassen, N.; Frolich, J.C. Development, validation and biomedical applications of stable-isotope dilution GC-MS and GC-MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15(S)-8-iso-prostaglandin F2alpha and nitric oxide (NO). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1019, 95–111. [Google Scholar]
- Mustieles, V.; Arrebola, J.P. How polluted is your fat? What the study of adipose tissue can contribute to environmental epidemiology. J. Epidemiol. Community Health 2020, 74, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Bertrand, K.A.; Franke, A.A.; Rosner, B.; Curhan, G.C.; Willett, W.C. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am. J. Clin. Nutr. 2017, 105, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Vernet, C.; Philippat, C.; Agier, L.; Calafat, A.M.; Ye, X.; Lyon-Caen, S.; Hainaut, P.; Siroux, V.; Schisterman, E.F.; Slama, R. An Empirical Validation of the Within-subject Biospecimens Pooling Approach to Minimize Exposure Misclassification in Biomarker-based Studies. Epidemiology 2019, 30, 756–767. [Google Scholar] [CrossRef]
- Johns, L.E.; Cooper, G.S.; Galizia, A.; Meeker, J.D. Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 2015, 85, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Knaak, J.B.; Sullivan, L.J. Metabolism of bisphenol A in the rat. Toxicol. Appl. Pharmacol. 1966, 8, 175–184. [Google Scholar] [CrossRef]
- Kovacic, P. How safe is bisphenol A? Fundamentals of toxicity: Metabolism, electron transfer and oxidative stress. Med. Hypotheses 2010, 75, 1–4. [Google Scholar] [CrossRef]
- Sakuma, S.; Nakanishi, M.; Morinaga, K.; Fujitake, M.; Wada, S.; Fujimoto, Y. Bisphenol A 3,4-quinone induces the conversion of xanthine dehydrogenase into oxidase in vitro. Food Chem. Toxicol. 2010, 48, 2217–2222. [Google Scholar] [CrossRef]
- Yoshida, M.; Ono, H.; Mori, Y.; Chuda, Y.; Onishi, K. Oxidation of bisphenol A and related compounds. Biosci. Biotechnol. Biochem. 2001, 65, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef] [PubMed]
| Study Design (Country) | Population Groups (Numbers of Participants) | Bisphenols (Source) | Oxidative Stress Effect Biomarkers (Source) | Outcomes | Reference |
|---|---|---|---|---|---|
| Diseases and health conditions | |||||
| Prospective cohort (Italy) | NAFLD * (n = 60) and controls (n = 60) living in Naples, subdivided in NAFL (n = 30)) and NASH (n = 30), investigated before/after BPA-free diet for 1 month | BPA (plasma and urine) | TBARS, SOD, CAT (all in serum) | The NAFLD patients had higher plasma (p < 0.0001) and urine (p < 0.0001) BPA levels as well as TBARS levels (p < 0.01; no separation step used) compared to controls. The plasma BPA levels were significantly higher in NASH patients than in NAFL patients (p = 0.041), independent of the presence of T2DM. SOD and CAT activities were higher in the NAFLD group than in controls (p < 0.01). After BPA-free diet, TBARS, SOD, and CAT levels were reduced, but not significantly | Dallio 2017 [14] |
| Case-control (China) | ADHD (n = 215) vs. healthy (n = 253) children aged 6‒12 years | BPA (urine) | 8-OHdG (urine) | ADHD children had significantly higher urinary BPA (p < 0.001) and 8-OHdG (p < 0.001) levels vs. controls, and 8-OHdG correlated positively with BPA (r = 0.257, p < 0.001) | Li 2018 [16] |
| Case-control (Egypt) | Children ASD (n = 49) vs. matched controls (n = 40) | BPA (serum) | 8-OHdG (serum) | Both BPA (p = 0.025) and 8-OHdG (p = 0.0001) were significantly higher in children with ASD, and there were highly significant positive correlations between both BPA (r = 0.400, p = 0.004) and 8-OHdG (r = 0.805, p = 0.001) with ASD severity | Metwally 2018 [17] |
| Case-control (Turkey) | Children with classic autism (n = 27), PDD-NOS (n = 10) and controls (n = 35) | BPA (plasma) | TBARS and protein carbonyls (plasma). GPx1, TrxR, CAT, SOD and GR activities, GSH, and Se levels (all in erythrocytes) | The PDD-NOS group had higher BPA levels than both control (p = 0.003) and classic autism groups (p = 0.003), but the classical autism group was not different from controls. Carbonyl levels were significantly higher in the study group (both classical autism and PDD-NOS) than controls (p = 0.025). Selenium levels and GPx1, SOD and GR activities were higher (p = 0.013; p = 0.002; p = 0.03; p < 0.001, respectively) and CAT activity was lower (p < 0.001) in the autistic group vs. controls. However, no correlations between BPA levels and antioxidant enzymes, antioxidants or oxidative stress effect biomarkers were found | Kondolot 2016 [18] |
| Case-control (Turkey) | COPD patients (n = 50) and controls (n = 33) | BPA (serum) | MDA and total thiols (serum) | In the serum of COPD patients, BPA concentrations were significantly higher (p < 0.001), and total thiol levels significantly lower (p = 0041), than in controls. Serum MDA did not differ between the groups | Erden 2014 [19] |
| Case-control (Egypt) | Infertile male patients (n = 50) vs. matched controls (n = 50) | BPA (urine) | Total antioxidant activity and MDA in seminal cell-free plasma | BPA concentrations were similar, MDA levels higher (p = 0.012) and total antioxidant levels lower (p = 0.001), in patients than controls. Overall (n = 100), BPA levels were positively correlated with seminal plasma lipid peroxidation (r = 0.298, p < 0.01) | Omran 2018 [20] |
| Intervention (single-blind randomized clinical trial) (South Korea) | Young women investigated for gynecological complaints given KRG (n = 11) or placebo (n = 11) | BPA (urine) | MDA (urine) | Urinary total BPA and MDA levels were positively associated (slope = 0.88, r2 = 0.10, p < 0.01). Intervention with KRG decreased both BPA (p < 0.05) and MDA (p < 0.05) levels | Yang 2014 [21] |
| Pregnant women and their fetuses/newborns | |||||
| Nested case-control with repeated measurements of exposure and outcomes during pregnancy (USA) | Pregnant women (n = 482), CRP, IL-1β, IL-6, IL-10 and TNF-α measured up to four times in plasma | BPA (urine) | 8-OHdG and 8-isoprostane (both urine), up to four measurements | In adjusted models, an IQR increase in BPA was associated with significant increases in oxidative stress biomarkers, 5% in 8-OHdG (p = 0.03) and 9% in 8-isoprostane (p = 0.02). Significantly higher IL-6 concentrations were associated with an IQR 8.95% increase in BPA | Ferguson 2016 [22] |
| Cohort with repeated measurements of exposure and outcomes during pregnancy (Puerto Rico) | Pregnant women (n = 106, of which 54 had oxidative stress biomarkers analyzed) | BPA (urine) | 8-OHdG and 8-isoprostane (both urine), three measurements | An IQR range increase in BPA was associated with 29% higher 8-isoprostane (p = 0.0002) and 21% higher 8-OHdG (p = 0.001) levels | Watkins 2015 [23] |
| Prospective pregnancy cohort (USA) | Pregnant women (n = 24), plasma and their umbilical cord at birth | uBPA and BPA-G (blood plasma) | 3-NO2Tyr, 3-ClTyr, and diTyr (all in plasma) | A positive association between BPA (as BPA-G: r = 0.440, p < 0.05; as uBPA: r = 0.398, p = 0.054 (not significant)) and 3-NO2Tyr levels (but not 3-ClTyr and diTyr) was found in plasma from pregnant mothers. In cord blood, 3-NO2Tyr levels were higher (p < 0.10) when mothers belonged to the highest uBPA exposed half | Veiga-Lopez 2015 [24] |
| Cross-sectional within a pregnancy cohort (Taiwan) | Study on mother/fetus pairs (n = 241 (244 in follow-up study) | BPA (urine) | 8-OHdG, 8-NO2Gua, 8-isoprostane and HNE-MA levels in 3rd trimester maternal urine. GPx in maternal and umbilical cord blood plasma | Positive associations between maternal BPA and 8-isoprostane levels (β = 4.5, p = 0.05), but no associations between BPA and 8-OHdG, HNE-MA or 8-NO2Gua levels [25,26]. BPA concentrations were inversely associated with maternal (β = −30.98, p = 0.04) and cord blood (β = −29.40, p = 0.01) plasma GPx levels. BPA was inversely associated with penis length (β = −4.43 mm, p = 0.005) among boys who were born to mothers high in 8-isoprostane [26] | Huang 2017 [25] and 2018 [26] |
| Cross-sectional within a pregnancy cohort (Taiwan) | Pregnant women (n = 186) in northern Taiwan and birth outcomes after delivery | BPA (urine) | 8-OHdG, 8-NO2Gua, 8-isoprostane, and HNE-MA (all urinary) | A significant but weak correlation was observed between maternal BPA and 8-isoprostane (r = 0.17, p = 0.02), whereas no correlation was found between BPA and the other oxidative stress biomarkers | Chang 2019 [27] |
| Children and adolescents | |||||
| Longitudinal cohort with repeated measurements (China) | School children (n = 275), age 7‒11 years at first sampling | BPA, BPB, BPAF, BPAP, BPE, BPF, BPP, BPS, BPZ (all in urine) | 8-OHdG and 8-OHG (both in urine) | An IQR increase in BPA was associated with 12.9% (p < 0.001) increase in 8-OHdG and 19.4% (p < 0.001) increase in 8-OHG. An IQR increase in the sum of the dominant BPs (BPA, BPS, BPF, and BPAF) was associated with 17.4% (p < 0.001) increase in 8-OHdG and 25.9% (p < 0.001) increase in 8-OHG, respectively. BPS was positively associated with 8-OHG (p < 0.006), but not with 8-OHdG | Zhou 2019 [28] |
| Cross-sectional (Brazil) | Urban resident children (n = 300), 6‒14 years, from five geographic regions in Brazil | BPA, BPS, BPAP, BPB, BPP, BPF, BPAF, BPZ, and BPM (all in urine) | 8-OHdG (urine) | Significant association between urinary 8-OHdG and BPA (r = 0.261, p < 0.01) and a number of other non-BPs EDCs. Other bisphenols had low detection frequency. Co-exposure to 10 EDCs, including BPA, was associated with oxidative DNA damage | Rocha 2018 [29] |
| Cross-sectional (Italy) | School children aged 7‒19 years (n = 223) | GlcA-BPA (urine) | 8-isoprostane (urine) | A significant association between 8-isoprostane and GlcA-BPA was found, but only from ≥6 ng/mg creatinine (p < 0.001) | Bono 2019 [30] |
| Cross-sectional (China) | Children aged 3‒6 years (n = 96) | BPA (urine) | 8-OHdG (urine) | A significant positive association was found (r = 0.240, p = 0.016) between BPA and 8-OHdG levels after ln-transformation of unadjusted data | Lv 2016 [31] |
| Adults | |||||
| Cross-sectional (with repeated measurements) (China) | Healthy Chinese men (n = 11), urine measurements taken repeatedly over 3 months | BPA, BPF, BPS (all in urine) | 8-OHdG, 8-isoprostane, and HNE-MA (all in urine) | BPA was positively associated with 8-OHdG (r = 0.19, p < 0.001), HNE-MA (r = 0.10, p = 0.03), and 8-isoprostane (r = 0.10, p = 0.03), whereas BPF was positively associated with HNE-MA (r = 0.11, p = 0.01) and 8-isoprostane (r = 0.10, p = 0.02), but negatively (not significantly) associated with 8-OHdG (r = −0.05, p = 0.30) | Wang 2019 [32] |
| Cross-sectional (South Korea) | Urban residing adults (n = 960) | BPA (urine) | 8-OHdG and MDA (both in urine) | 8-OHdG and MDA levels were not significantly associated with BPA in Korean urban residing adults | Hong 2009 [33] |
| Cross-sectional (South Korea) | Adult men (n = 259), pre- (n = 92) and postmenopausal (n = 134) women | BPA (urine) | 8-OHdG and MDA (urine) | BPA was positively associated with MDA (β = 0.056‒0.066, p = 0.006‒0.008 in 3 of 3 models) and 8-OHdG (β = 0.072‒0.103, p = 0.008‒0.025 in 3 of 3 models) in postmenopausal women, but not in men and premenopausal women | Yang 2009 [34] |
| Cross-sectional (Saudi Arabia) | Healthy persons (n = 130), aged 1‒87 years | BPA, BPAF, BPAP, BPS, BPF, BPP, BPZ, and BPB (all in urine) | 8-OHdG (urine) | BPA (r = 0.38, p < 0.0001) and BPS (r = 0.30, p < 0.0005) were significantly associated with 8-OHdG levels. All 8 BPs together were positively associated (r = 0.43, p < 0.0001) with 8-OHdG levels | Asimakopoulos 2016 [35] |
| Cross-sectional (Singapore) | Healthy participants (n = 33), 22‒37 years | BPA, BPS, several BADGEs (urine) | 8-OHdG (urine) | 8-OHdG levels were positively correlated with BPA levels (r = 0.353, p < 0.05) | Liu 2019 [36] |
| Cross-sectional (South Korea) | General population (n = 585 persons for urine, n = 465 for serum, aged 20‒70 years) | BPA (serum and urine) | bilirubin (serum) | An inverse association between urinary BPA and serum bilirubin levels (β = −0.071, p < 0.0001) was found, whereas no association was found between serum BPA and serum bilirubin | Choi 2019 [37] |
| Intervention (South Korea) | Young women (n = 14) given wheat sprout juice for 14 days | BPA (urine) | 8-OHdG and MDA (urine) | BPA concentrations were positively associated with MDA levels (slope = 0.85, p = 0.03) and 8-OHdG (slope = 1.47, p = 0.18 (not significant)) | Yi 2011 [38] |
| Elderly | |||||
| Prospective cohort with repeated measurements (South Korea) | Elderly (n = 471) investigated for liver function and polymorphisms in oxidative stress-related genes | BPA (urine) | SNPs in COX2, EPHX1, CAT and SOD2 genes in blood cells | Significant associations of BPA with abnormal liver function were found in Koreans with certain polymorphisms in genes associated with oxidative stress, such as COX2, CAT, EPHX1, and SOD2 | Kim 2016 [39] |
| Prospective cohort with repeated measurements (South Korea) | Elderly (n = 548) investigated for polymorphisms in oxidative stress-related genes and oxidative stress | BPA (urine) | SNPs in COX2, EPHX1, CAT, SOD2, HSP70-hom, PON1, eNOS, DRD2, and MPO genes and MDA (urine) | A significant positive association was found between urinary BPA and MDA in both sexes (males: β = 0.19 and p = 0.0003, females: β = 0.18 and p < 0.0001) regardless of any genotype of the nine oxidative stress-related genes | Kim and Hong 2017 [40] |
| Exposures based on residence area | |||||
| Cross-sectional (China) | Residents (n = 116), 0.4‒87 years, living in the e-waste recycling region Longtang Town, Qingyuan City, or in rural or urban reference areas | BPA, BPAF, BPAP, BPS, BPF, BPP, BPZ, and BPB (all in urine) | 8-OHdG (urine) | In the e-waste dismantling location, urinary BPA (r = 0.413, p < 0.001) and BPS (r = 0.386, p < 0.001), but not BPF (r = 0.118, p = 0.208), were positively associated with urinary 8-OHdG levels. Similar correlations were also observed for the combined data from the two reference areas | Zhang 2016 [41] |
| POSITIVE | NO ASSOCIATION | NEGATIVE 1 |
|---|---|---|
| 8-OHdG 2 | ||
| Li 2018 (urine) 3 Metwally 2018 (serum) Zhou 2019 (urine) Ferguson 2016 (urine) Watkins 2015 (urine) Yang 2009 (urine; only in postmenopausal women) Wang 2019 (urine) Asimakopoulos 2016 (urine) Liu 2019 (urine) Rocha 2018 (urine) Zhang 2016 (urine) | Huang 2017 and 2018, and Chang 2019 (urine) Lv 2016 (urine; POS in µg/L when unadjusted, but not as µg/L after creatinine normalization, POS as ln of µg/L) Hong 2009 (urine) | - |
| 8-isoprostane | ||
| Ferguson 2016 (urine) Watkins 2015 (urine) Huang 2017 and 2018, and Chang 2019 (urine) Bono 2019 (urine; POS at ≥6 ng/mL creatinine) Wang 2019 (urine) | - | - |
| MDA (chromatographic separation) | ||
| Yang 2014 (urine) Yang 2009 (urine; only in postmenopausal women) Yi 2011 (urine) Kim and Hong 2017 (urine) | Hong 2009 (urine) | |
| MDA (unspecific detection) and other aldehydes using TBARS | ||
| Dallio 2018 (serum TBARS) Omran 2018 (seminal plasma) | Kondolot 2016 (plasma TBARS) Erden 2014 (serum) | - |
| HNE-MA | ||
| Wang 2019 (urine) | Huang 2017 and 2018, and Chang 2019 (urine) | - |
| 8-NO2Gua | ||
| - | Huang 2017 and 2018, and Chang 2019 (urine) | - |
| Antioxidant enzymes | ||
| Dallio 2018 (serum SOD and CAT) | Kondolot 2016 (erythrocytes) | Huang 2017 (maternal plasma and cord blood (GPx) |
| Total thiols and GSH | ||
| - | Kondolot 2016 (erythrocytes) | - |
| - | Erden 2014 (serum) | - |
| Oxidative stress genetic polymorphism (DNA) | ||
| Kim 2016 (association BPA vs. liver function for certain oxidative stress genes) | - | - |
| 3-NO2Tyr | ||
| Veiga-Lopez 2015 (plasma) | - | - |
| 3-ClTyr (plasma) and diTyr | ||
| - | Veiga-Lopez 2015 (plasma) | - |
| 8-OHG | ||
| Zhou 2019 (urine) | - | - |
| Bilirubin (serum) | ||
| - | Choi 2019 (serum) | Choi 2019 (urine) |
| Protein carbonyls | ||
| - | Kondolot 2016 (plasma) | - |
| Total antioxidant activity (no significant outcomes) | ||
| - | - | - |
| Biomarker (Source) | Number of Human Studies 1 | Associations between BPA and Oxidative Stress Biomarkers | Suitable Analytical Methods | Strengths | Weaknesses |
|---|---|---|---|---|---|
| 8-OHdG 2 (urine) | 15 | Dominantly positive associations, but also equivocal results. No negative associations | Best options: LC-MS or HPLC-EC. Other options: ELISA, GC-MS | Easy to measure | Not BPA- or organ-specific. May originate from food or endogenous sources. Can display considerable inter- and intra-day variations |
| 8-isoprostane (urine) | 5 | Only positive associations | Best options: LC-MS or GC-MS | 8-isoprostane is specific for lipid peroxidation | Not BPA- or organ-specific |
| MDA (chromatographic separation) (urine) | 5 | Dominantly positive associations, but also equivocal results. No negative associations | Best option: derivatization followed by GC-MS or HPLC separation prior to UV or fluorescence analysis | MDA is specific for lipid peroxidation | Risk of artefactual generation during preparatory steps involving excessive heating prior to analysis. Not BPA- or organ-specific |
| MDA (unspecific detection) and other aldehydes using TBARS (serum, plasma) | 4 | Two positive, two with no associations | Not recommended: UV or fluorescence analysis of formed chromophore, but no separatory step used in the analysis | May be related to oxidative stress | Unclear what is measured. Risk of artefactual generation during preparatory steps. Not BPA- or organ-specific |
| Antioxidant enzymes (erythrocytes, plasma, serum) | 3 | Heterogeneous outcomes | Best option: enzymatic activity assay. Others: Western blot or ELISA | Antioxidant enzymes may be upregulated during conditions of oxidative stress | Not BPA- or organ-specific. High oxidative stress levels may be required to induce additional synthesis of antioxidant enzymes |
| HNE-MA (urine) | 2 | One positive, one with no association | Best options: LC-MS, ELISA | HNE-MA is specific for lipid peroxidation | Not BPA- or organ-specific |
| Total thiols and GSH (erythrocytes, serum) | 2 | No associations | Best option: LC-MS. Others: kinetic assay or by spectrophotometry | Lowered SH groups may be related to increased oxidative stress | Not BPA- or organ-specific. GSH is used in a broad range of biochemical reactions making it less specific |
| Polymorphisms in oxidative stress genes (DNA) | 2 (1 for liver function, 1 for MDA using separation) | Both studies found positive associations | Best option: DNA gene sequencing using PCR | Poly-morphisms in oxidative stress genes may be related to susceptibility to toxic substances and diseases | Not BPA- or organ-specific. Protein levels may not always reflect the number of gene copies. Proteins can have several functions |
| 3-NO2Tyr/3-ClTyr/diTyr (plasma) | 1 | Positive association for 3-NO2Tyr. No association for 3-ClTyr or diTyr | Best option: LC-MS | 3-NO2Tyr, 3-ClTyr and diTyr are specific for RNS, HOCl and ROS, respectively | Not BPA- or organ-specific |
| 8-OHG | 1 | Positive association | Best options: LC-MS or HPLC-EC. Others: ELISA, GC-MS | Easy to measure | Not BPA- or organ-specific. May originate from food or endogenous sources. Can display considerable inter- and intra-day variations |
| 8-NO2Gua (urine) | 1 | No association | Best option: LC-MS | 8-NO2Gua is specific for RNS | Not BPA- or organ-specific |
| Protein carbonyls (plasma) | 1 | No association | Best option: ELISA | Protein carbonyls are specific for oxidative stress and are considered stable being only slowly repaired/turned over | Not BPA- or organ-specific. Origin somewhat unclear |
| Bilirubin (serum and urine) | 1 | Inverse association (urine) and no association (serum) | Best option: LC-MS | Easy to measure | Not BPA- or organ-specific |
| Total antioxidant activity (seminal plasma) | 1 | No association | Best option: commercial H2O2-based kit | Easy to measure | Not BPA- or organ-specific. Unclear what is measured |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffensen, I.-L.; Dirven, H.; Couderq, S.; David, A.; D’Cruz, S.C.; Fernández, M.F.; Mustieles, V.; Rodríguez-Carrillo, A.; Hofer, T. Bisphenols and Oxidative Stress Biomarkers—Associations Found in Human Studies, Evaluation of Methods Used, and Strengths and Weaknesses of the Biomarkers. Int. J. Environ. Res. Public Health 2020, 17, 3609. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103609
Steffensen I-L, Dirven H, Couderq S, David A, D’Cruz SC, Fernández MF, Mustieles V, Rodríguez-Carrillo A, Hofer T. Bisphenols and Oxidative Stress Biomarkers—Associations Found in Human Studies, Evaluation of Methods Used, and Strengths and Weaknesses of the Biomarkers. International Journal of Environmental Research and Public Health. 2020; 17(10):3609. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103609
Chicago/Turabian StyleSteffensen, Inger-Lise, Hubert Dirven, Stephan Couderq, Arthur David, Shereen Cynthia D’Cruz, Mariana F Fernández, Vicente Mustieles, Andrea Rodríguez-Carrillo, and Tim Hofer. 2020. "Bisphenols and Oxidative Stress Biomarkers—Associations Found in Human Studies, Evaluation of Methods Used, and Strengths and Weaknesses of the Biomarkers" International Journal of Environmental Research and Public Health 17, no. 10: 3609. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17103609





