Multicomponent Exercise Program Reduces Frailty and Inflammatory Biomarkers and Improves Physical Performance in Community-Dwelling Older Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
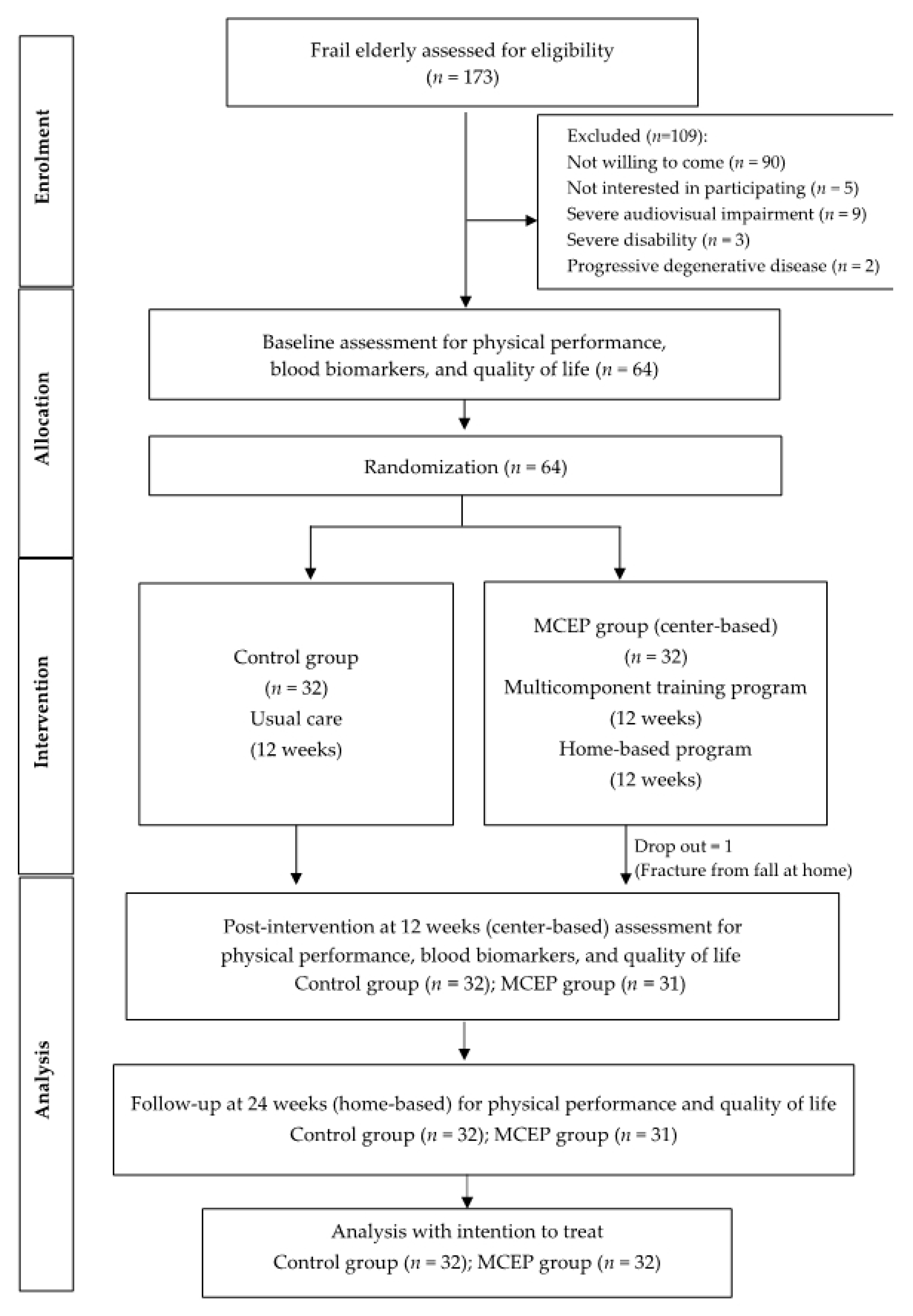
2.1. Study Design and Participants
2.2. Intervention Program
2.3. Outcome Measurements
2.4. Statistical Analysis
2.5. Ethical Consideration
3. Results
3.1. Baseline Descriptive Data
3.2. Changes in Physical Performance During the 24-Weeks Intervention Period
3.3. Changes in Frailty Score and HRQOL During the 24-Weeks Intervention Period
3.4. Changes in Blood Biomarker During the 12-Weeks Intervention Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- John, K.; Napaporn, C. Inter-generational family care for and by older people in Thailand. Int. J. Sociol. Soc. Policy 2012, 32, 682–694. [Google Scholar]
- National Statistical Office. Report on the 2017 Survey of the Older Persons in Thailand; Statistical Forecasting Division, National Statistical Office: Bangkok, Thailand, 2018.
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.G.M.O.; Rockwood, K. Frailty in Older People Summary. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Vellas, B.; Hsu, F.-C.; Newman, A.B.; Doss, H.; King, A.C.; Manini, T.M.; Church, T.; Gill, T.M.; Miller, M.E.; et al. A Physical Activity Intervention to Treat the Frailty Syndrome in Older Persons—Results From the LIFE-P Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 70, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.; Wall, J.; Biram, R.; Romero-Ortuño, R. Association of the clinical frailty scale with hospital outcomes. QJM 2015, 108, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmarath, W.; Seesen, M.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Siviroj, P.; Limtrakul (Dejkriengkraikul), P. The association between frailty indicators and blood-based biomarkers in early-old community dwellers of Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3457. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [Green Version]
- Puts, M.T.E.; Visser, M.; Twisk, J.W.R.; Deeg, R.J.H.; Lips, P. Endocrine and inflammatory markers as predictors of frailty. Clin. Endocrinol. 2005, 63, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Pérez, D.; Sánchez-Flores, M.; Maseda, A.; Lorenzo-López, L.; Millán-Calenti, J.C.; Gostner, J.M.; Fuchs, D.; Pásaro, E.; Laffon, B.; Valdiglesias, V. Frailty in older adults is associated with plasma concentrations of inflammatory mediators but not with lymphocyte subpopulations. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.T.; Nguyen, T.X.; Nguyen, T.N.; Nguyen, T.H.T.; Pham, T.; Cumming, R.; Hilmer, S.N.; Vu, H.T.T. The impact of frailty on prolonged hospitalization and mortality in elderly inpatients in Vietnam: A comparison between the frailty phenotype and the Reported Edmonton Frail Scale. Clin. Interv. Aging 2019, 14, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Impact of physical frailty on disability in community-dwelling older adults: A prospective cohort study. BMJ Open 2015, 5, e008462. [Google Scholar] [CrossRef]
- Seesen, M.; Semmarath, W.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Ongprasert, K.; Chittrakul, J.; Siviroj, P.; Limtrakul (Dejkriengkraikul), P. Combined black rice germ, bran supplement and exercise intervention modulate aging biomarkers and improve physical performance and lower-body muscle strength parameters in aging population. Int. J. Environ. Res. Public Health 2020, 17, 2931. [Google Scholar] [CrossRef] [PubMed]
- De Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydwik, E.; Frändin, K.; Akner, G. Effects of physical training on physical performance in institutionalised elderly patients (70+) with multiple diagnoses. Age Ageing 2004, 33, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheerman, K.; Raaijmakers, K.; Otten, R.H.; Meskers, C.G.M.; Maier, A.B. Effect of physical interventions on physical performance and physical activity in older patients during hospitalization: A systematic review 11 Medical and Health Sciences 1117 Public Health and Health Services. BMC Geriatr. 2018, 18, 288. [Google Scholar]
- Chittrakul, J.; Siviroj, P.; Sungkarat, S.; Sapbamrer, R. Multi-System Physical Exercise Intervention for Fall Prevention and Quality of Life in Pre-Frail Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3102. [Google Scholar] [CrossRef]
- Lee, P.G.; Jackson, E.A.; Richardson, C.R. Exercise prescriptions in older adults. Am. Fam. Physician 2017, 95, 425–432. [Google Scholar]
- Riebe, D. General principles of exercise prescription. In ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The Effectiveness of Exercise Interventions for the Management of Frailty: A Systematic Review. J. Aging Res. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Silpakit, O.; Silpakit, C.; Ph, D.; Pukdeenaul, P. A Comparison Study of Cognitive Impairment Screening Tools: CDR, IQCODE VS MMSE. Siriraj Med. J. 2007, 59, 2002–2004. [Google Scholar]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodríguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. AGE 2014, 36, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, R.S.; Tanaka, H. Exercise prescription for the elderly: Current recommendations. Sports Med. 2001, 31, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Best-Martini, E.; Jones-DiGenova, K.A. Exercise For Frail Elders, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2014. [Google Scholar]
- Tarazona-Santabalbina, F.J.; Gomez-Cabrera, M.C.; Pérez-Ros, P.; Martínez-Arnau, F.M.; Cabo, H.; Tsaparas, K.; Salvador-Pascual, A.; Rodríguez-Mañas, L.; Viña, J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2016, 17, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsani, A.A.; Spina, R.J.; Peterson, L.R.; Rinder, M.R.; Glover, K.L.; Villareal, D.T.; Binder, E.F.; Holloszy, J.O. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J. Appl. Physiol. 2003, 95, 1781–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, K.; Robinson, K.; Logan, P.; Gordon, A.L.; Harwood, R.H.; Masud, T. Chair-based exercises for frail older people: A systematic review. BioMed Res. Int. 2013, 2013, 309506. [Google Scholar] [CrossRef] [PubMed]
- Arena, S. Rate Perceived Exertion as a Measure of Exercise Intensity. Home Health Now 2017, 35, 570. [Google Scholar] [CrossRef]
- Campbell, A.J.; Robertson, M.C. Otago Exercise Programme to Prevent Falls in Older Adults: A Home-Based, Individually Tailored Strength and Balance Retraining Programme; ACC: Mumbai, India, 2003; pp. 1–72. [Google Scholar]
- Schaap, L.; Fox, B.; Henwood, T.; Bruyère, O.; Reginster, J.; Beaudart, C.; Buckinx, F.; Roberts, H.C.; Cooper, C.; Cherubini, A.; et al. Grip strength measurement: Towards a standardized approach in sarcopenia research and practice. Eur. Geriatr. Med. 2016, 7, 247–255. [Google Scholar] [CrossRef]
- Thorbahn, L.D.B.; A Newton, R. Use of the Berg Balance Test to Predict Falls in Elderly Persons. Phys. Ther. 1996, 76, 576–583. [Google Scholar] [CrossRef]
- Gadelha, A.B.; Neri, S.G.R.; De Oliveira, R.J.; Bottaro, M.; De David, A.C.; Vainshelboim, B.; Lima, R.M. Severity of sarcopenia is associated with postural balance and risk of falls in community-dwelling older women. Exp. Aging Res. 2018, 44, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Tillin, T.; Williams, S.; Coady, E.; Chaturvedi, N.; Hughes, A.D. Assessment of exercise capacity and oxygen consumption using a 6 min stepper test in older adults. Front. Physiol. 2017, 8, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Global Strategy on Diet, Physical Activity and Health. Physical Activity and Young People; World Health Organization: Geneva, Switzerland, 2010; pp. 1–2.
- Ware, J.E.; Sherbourne, C.D. The MOS 36-ltem Short-Form Health Survey (SF-36). Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.E.; Elangovan, N.; Yeh, I.L.; Konczak, J. The effectiveness of proprioceptive training for improving motor function: A systematic review. Front. Hum. Neurosci. 2015, 8, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadore, E.L.; Rodrı, L. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults: A Systematic Review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, E.F.; Schechtman, K.B.; Ehsani, A.A.; Steger-May, K.; Brown, M.; Sinacore, D.R.; Yarasheski, K.; Holloszy, J.O. Effects of exercise training on frailty in community-dwelling older adults: Results of a randomized, controlled trial. J. Am. Geriatr. Soc. 2002, 50, 1921–1928. [Google Scholar] [CrossRef]
- Justine, M.; Hamid, T.A.; Mohan, V.; Jagannathan, M. Effects of Multicomponent Exercise Training on Physical Functioning among Institutionalized Elderly. ISRN Rehabil. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cadore, E.L.; Pinto, R.S.; Bottaro, M.; Izquierdo, M. Strength and Endurance Training Prescription in Healthy and Frail Elderly. Aging Dis. 2014, 5, 183–195. [Google Scholar] [CrossRef]
- Yoon, D.H.; Kang, D.; Kim, H.J.; Kim, J.S.; Song, H.S.; Song, W. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr. Gerontol. Int. 2017, 17, 765–772. [Google Scholar] [CrossRef]
- Fiatarone, M.A.; Marks, E.C.; Ryan, N.D.; Meredith, C.N.; Lipsitz, L.A.; Evans, W.J. High-Intensity Strength Training in Nonagenarians: Effects on Skeletal Muscle. JAMA J. Am. Med. Assoc. 1990, 263, 3029–3034. [Google Scholar] [CrossRef]
- Latham, N.K.; Bennett, D.A.; Stretton, C.S.; Anderson, C.A. Systematic Review of Progressive Resistance Training in Older Adults. J. Geriatr. Phys. Ther. 2002, 25, 28–29. [Google Scholar] [CrossRef]
- Cadore, E.L.; Pinto, R.S.; Pinto, S.S.; Alberton, C.L.; Correa, C.; Tartaruga, M.P.; Silva, E.M.; Almeida, A.P.V.; Trindade, G.T.; Kruel, L.F.M. Effects of Strength, Endurance, and Concurrent Training on Aerobic Power and Dynamic Neuromuscular Economy in Elderly Men. J. Strength Cond. Res. 2011, 25, 758–766. [Google Scholar] [CrossRef]
- Jankord, R.; Jemiolo, B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med. Sci. Sports Exerc. 2004, 36, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tan, C.T.Y.; Nyunt, M.S.Z.; Mok, E.W.H.; Camous, X.; Kared, H.; Fulop, T.; Feng, L.; Ng, T.P.; Larbi, A. Inflammatory and immune markers associated with physical frailty syndrome: Findings from Singapore longitudinal aging studies. Oncotarget 2016, 7, 28783–28795. [Google Scholar] [CrossRef] [PubMed]
- Ershler, W.B.; Keller, E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Coral Reefs 2000, 51, 245–270. [Google Scholar]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Papini, C.B.; Nakamura, P.M.; Zorzetto, L.P.; Thompson, J.L.; Phillips, A.C.; Kokubun, E. The effect of a community-based, primary health care exercise program on inflammatory biomarkers and hormone levels. Mediat. Inflamm. 2014, 2014, 185707. [Google Scholar] [CrossRef]
- Ferreira, C.B.; Teixeira, P.D.S.; Dos Santos, G.A.; Maya, A.T.D.; Brasil, P.A.D.; Souza, V.C.; Córdova, C.; Ferreira, A.; Lima, R.M.; Nóbrega, O.D.T. Effects of a 12-Week Exercise Training Program on Physical Function in Institutionalized Frail Elderly. J. Aging Res. 2018, 2018, 7218102. [Google Scholar] [CrossRef] [Green Version]
- Bray, N.W.; Smart, R.R.; Jakobi, J.M.; Jones, G.R. Exercise prescription to reverse frailty. Appl. Physiol. Nutr. Metab. 2016, 41, 1112–1116. [Google Scholar] [CrossRef] [Green Version]
- Sialino, L.D.; Schaap, L.A.; Van Oostrom, S.H.; Nooyens, A.C.J.; Picavet, H.S.J.; Twisk, J.W.R.; Verschuren, W.M.M.; Visser, M.; Wijnhoven, H.A.H. Sex differences in physical performance by age, educational level, ethnic groups and birth cohort: The Longitudinal Aging Study Amsterdam. PLoS ONE 2019, 14, e0226342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.J.; Lee, K.; Kim, B.H. Gender differences in the association between frailty, cognitive impairment, and self-care behaviors among older adults with atrial fibrillation. Int. J. Environ. Res. Public Health 2019, 16, 2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsekoura, M.; Billis, E.; Tsepis, E.; Dimitriadis, Z.; Matzaroglou, C.; Tyllianakis, M.; Panagiotopoulos, E.; Gliatis, J. The Effects of Group and Home-Based Exercise Programs in Elderly with Sarcopenia: A Randomized Controlled Trial. J. Clin. Med. 2018, 7, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, R.; Miura, Y. The effect of exercise intervention on frail elderly in need of care: Half-day program in a senior day-care service facility specializing in functional training. J. Phys. Ther. Sci. 2016, 28, 1957–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenardt, M.H.; Carneiro, N.H.K.; Albino, J.; Willig, M.H. Quality of life of frail elderly users of the primary care. Acta Paul. Enferm. 2014, 27, 399–404. [Google Scholar] [CrossRef] [Green Version]

| Exercise Training | Description | Duration | Intensity Progression |
|---|---|---|---|
| Chair Aerobic Training | (i) seated marching, (ii) leg marching, (iii) arm swing, (iv) tap and clap, (v) side bend, and (vi) arm raised | 10−20 min | month 1: 10 min month 2: 15 min month 3: 20 min |
| Resistance Training with Theraband | (i) arm curl, (ii) backward arm press, (iii) hip flexor, (iv) hip extensor, (v) hip adductor, (vi) hip abductor, (vii) knee flexor, (viii) knee extensor, (ix) ankle plantar flexor, and (x) ankle dorsiflexor | 25−30 min | month 1, Reps: 8 × 2 set, intensity: 65% of the 1RM month 2, Reps: 10 × 3 set, intensity: 75% of the 1RM month 3, Reps: 12 × 3 set, intensity: 85−90% of the 1RM (Intensity was set by the color of the theraband.) |
| Balance Training | (i) sit to stand, (ii) knee bends, (iii) backwards walking, (iv) walking and turning around, (v) sideways walking, and (vi) heel toe standing (vii) heel toe walking (viii) one leg stand | 10 min | Month 1: two hands support month 2: one hand support month 3: no hand support |
| Characteristics | Control Group (n = 32) | MCEP Group (n = 32) | p-Value |
|---|---|---|---|
| Age (y), mean ± SD | 78.87 ± 1.32 | 76.68 ± 1.14 | 0.64 |
| Sex, n (%) | |||
| Female | 16 (50.0) | 23 (71.9) | 0.06 |
| Male | 16 (50.0) | 9 (28.1) | |
| Number of Comorbidities, mean ± SD | 1.25 ± 0.76 | 1.09 ± 0.96 | 0.35 |
| Number of Types of Medication, mean ± SD | 2.62 (1.91) | 2.28 ± 1.97 | 0.67 |
| Self-health Rating, n (%) | |||
| Excellent | 4 (12.5) | 6 (18.8) | 0.61 |
| Good | 19 (59.4) | 16 (50.0) | |
| Fair / poor | 9 (28.1) | 10 (31.2) | |
| BMI, mean ± SD | 21.28 (0.69) | 21.37 (0.68) | 0.84 |
| HRQOL Score (range, 0−100), mean ± SD | 60.77 (16.84) | 61.45 (13.3) | 0.27 |
| Frailty Score, mean ± SD | 3.25 (0.50) | 3.18 (0.39) | 0.58 |
| Frailty Criteria, n (%) | |||
| Weight Loss | 8 (25.0) | 6 (18.8) | 0.76 |
| Low Grip Strength | 31 (96.9) | 29 (90.6) | 0.61 |
| Low Walking Speed | 25 (78.1) | 24 (75) | 1.00 |
| Exhaustion | 15 (46.9) | 17 (53.1) | 0.84 |
| Low Physical Activity | 29 (90.6) | 32 (100.0) | 0.27 |
| Parameters | Control Group (n = 32) | MCEP Group (n = 32) | Group × Time # | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 12-Weeks | 24-Weeks | Baseline | 12-Weeks | 24-Weeks | p | |
| Physical Performance | |||||||
| Strength (Handgrip (kg)) | 15.50 ± 6.47 | 16.28 ± 7.00 | 16.70 ± 8.05 | 15.71 ± 6.21 | 19.56 ± 5.27 *,† | 18.84 ± 5.01 † | 0.08 |
| Berg Balance Score | 49.96 ± 4.40 | 45.34 ± 8.65 | 44.46 ± 9.52 † | 49.12 ± 3.58 | 53.12 ± 3.16 *,† | 52.68 ± 3.49 *,† | <0.01 |
| TUG (sec) | 12.43 ± 5.04 | 15.57 ± 7.65 † | 15.75 ± 6.96 † | 12.21 ± 2.26 | 10.48 ± 2.16 *,† | 10.33 ± 2.91 *,† | <0.01 |
| VO2Max | 26.85 ± 4.57 | 26.12 ± 4.60 | 25.92 ± 4.07 | 26.53 ± 3.14 | 28.69 ± 4.39 * | 27.59 ± 4.14 | 0.07 |
| Frailty Score | 3.25 ± 0.50 | 3.03 ± 1.20 | 3.09 ± 0.92 | 3.18 ± 0.39 | 1.59 ± 0.83 *,† | 1.65 ± 0.86 *,† | <0.01 |
| HRQOL; SF-36 | |||||||
| Overall | 61.28 ± 16.17 | 60.49 ± 21.63 | 56.69± 13.34 | 61.90 ± 13.23 | 61.79 ± 20.92 | 59.56 ± 14.00 | 0.58 |
| PCS | 53.56 ± 17.83 | 52.53 ± 20.43 | 52.82 ± 20.98 | 53.38 ± 14.28 | 64.88 ± 22.96 † | 60.78 ± 22.5 | 0.17 |
| MCS | 73.88 ± 17.41 | 70.68 ± 21.94 | 69.36 ± 20.28 | 71.11 ± 15.87 | 74.14 ± 21.79 | 72.81 ± 21.71 | 0.25 |
| Blood Biomarker | Control Groupleft (n = 32) | MCEP Groupleft (n = 32) | Group × Time # | ||
|---|---|---|---|---|---|
| Baseline | 12-Weeks | Baseline | 12-Weeks | p | |
| IL-6 | 11.93 ± 17.56 | 11.04 ± 8.93 | 10.15 ± 6.25 | 8.16 ± 8.58 † | 0.005 |
| CRP | 3.97± 5.82 | 4.60 ± 6.91 | 3.83 ± 5.13 | 2.49 ± 4.46 † | 0.022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadjapong, U.; Yodkeeree, S.; Sungkarat, S.; Siviroj, P. Multicomponent Exercise Program Reduces Frailty and Inflammatory Biomarkers and Improves Physical Performance in Community-Dwelling Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3760. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17113760
Sadjapong U, Yodkeeree S, Sungkarat S, Siviroj P. Multicomponent Exercise Program Reduces Frailty and Inflammatory Biomarkers and Improves Physical Performance in Community-Dwelling Older Adults: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2020; 17(11):3760. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17113760
Chicago/Turabian StyleSadjapong, Uratcha, Supachai Yodkeeree, Somporn Sungkarat, and Penprapa Siviroj. 2020. "Multicomponent Exercise Program Reduces Frailty and Inflammatory Biomarkers and Improves Physical Performance in Community-Dwelling Older Adults: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 17, no. 11: 3760. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17113760






