Impact of Molar Furcations on Photodynamic Therapy Outcomes: A 6-Month Split-Mouth Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Inclusion/Exclusion Criteria
- (a)
- at least 40 years-old;
- (b)
- at least 20 teeth (third molars not included);
- (c)
- at least 30% of sites with CAL > 5 mm and ≥5 sites with PPD ≥ 5 mm for each quadrant;
- (d)
- at least one molar per quadrant (third molars not included);
- (e)
- bone loss;
- (f)
- bleeding on probing (BOP) ≥ 30%.
- (a)
- aggressive periodontitis [34];
- (b)
- smokers with more than 10 cigarettes/day;
- (c)
- antibiotic and anti-inflammatory treatments in the last six months;
- (d)
- previous periodontal therapy;
- (e)
- medical history likely to affect periodontal status and/or to compromise treatment outcomes;
- (f)
- pregnant/breastfeeding patients.
2.2. Clinical Measurements
2.3. Randomization
2.4. Study Design and Treatments
2.5. Examiner Calibration
2.6. Calculation of Sample Size
2.7. Statistical Analyses
3. Results
3.1. Characteristics of Studied Population
3.2. Initial Periodontal Parameters and Treatment Outcomes at Molar Furcation Sites Versus Other Sites in Test (SRP + PDT) and Control (SRP) Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heitz-Mayfield, L.J.A.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontology 2000 2013, 62, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Leyland, A.H.; Wennström, J.L. Factors influencing the outcome of non-surgical periodontal treatment: A multilevel approach. J. Clin. Periodontol. 2007, 34, 682–690. [Google Scholar] [CrossRef]
- Svärdström, G.; Wennström, J.L. Periodontal treatment decisions for molars: An analysis of influencing factors and long-term outcome. J. Periodontol. 2000, 71, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Nordland, P.; Garrett, S.; Kiger, R.; Vanooteghem, R.; Hutchens, L.H.; Egelberg, J. The effect of plaque control and root debridement in molar teeth. J. Clin. Periodontol. 1987, 14, 231–236. [Google Scholar] [CrossRef]
- Loos, B.; Nylund, K.; Claffey, N.; Egelberg, J. Clinical effects of root debridement in molar and non-molar teeth. A 2-year follow-up. J. Clin. Periodontol. 1989, 16, 498–504. [Google Scholar] [CrossRef]
- Van der Weijden, G.A.F.; Dekkers, G.J.; Slot, D.E. Success of non-surgical periodontal therapy in adult periodontitis patients: A retrospective analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, C.; Wennström, J.L. Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets: Outcome at furcation sites. J. Periodontol. 2011, 82, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; De Buitrago, J.G.; Reddy, M.S. Periodontal regeneration—furcation defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, 108–130. [Google Scholar] [CrossRef] [Green Version]
- Salvi, G.E.; Mischler, D.C.; Schmidlin, K.; Matuliene, G.; Pjetursson, B.E.; Brägger, U.; Lang, N.P. Risk factors associated with the longevity of multi-rooted teeth. Long-term outcomes after active and supportive periodontal therapy. J. Clin. Periodontol. 2014, 41, 701–707. [Google Scholar] [CrossRef]
- Graetz, C.; Schützhold, S.; Plaumann, A.; Kahl, M.; Springer, C.; Sälzer, S.; Holtfreter, B.; Kocher, T.; Dörfer, C.E.; Schwendicke, F. Prognostic factors for the loss of molars—an 18-years retrospective cohort study. J. Clin. Periodontol. 2015, 42, 943–950. [Google Scholar] [CrossRef]
- Dannewitz, B.; Zeidler, A.; Hüsing, J.; Saure, D.; Pfefferle, T.; Eickholz, P.; Pretzl, B. Loss of molars in periodontally treated patients: Results 10 years and more after active periodontal therapy. J. Clin. Periodontol. 2016, 43, 53–62. [Google Scholar] [CrossRef]
- Nibali, L.; Zavattini, A.; Nagata, K.; Di Iorio, A.; Lin, G.-H.; Needleman, I.; Donos, N. Tooth loss in molars with and without furcation involvement—A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 156–166. [Google Scholar] [CrossRef]
- Dannewitz, B.; Lippert, K.; Lang, N.P.; Tonetti, M.S.; Eickholz, P. Supportive periodontal therapy of furcation sites: Non-surgical instrumentation with or without topical doxycycline. J. Clin. Periodontol. 2009, 36, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Luchesi, V.H.; Pimentel, S.P.; Kolbe, M.F.; Ribeiro, F.V.; Casarin, R.C.; Nociti, F.H., Jr.; Sallum, E.A.; Casati, M.Z. Photodynamic therapy in the treatment of class II furcation: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 781–788. [Google Scholar] [CrossRef]
- Eickholz, P.; Nickles, K.; Koch, R.; Harks, I.; Hoffmann, T.; Kim, T.-S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; et al. Is furcation involvement affected by adjunctive systemic amoxicillin plus metronidazole? A clinical trials exploratory subanalysis. J. Clin. Periodontol. 2016, 43, 839–848. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Garcia Canas, P.; Khouly, I.; Sanz, J.; Loomer, P.M. Effectiveness of systemic antimicrobial therapy in combination with scaling and root planing in the treatment of periodontitis: A systematic review. J. Am. Dent. Assoc. 2015, 146, 150–163. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Graziani, F.; Gatto, R.; Monaco, A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 514–526. [Google Scholar] [CrossRef]
- Xue, D.; Tang, L.; Bai, Y.; Ding, Q.; Wang, P.; Zhao, Y. Clinical efficacy of photodynamic therapy adjunctive to scaling and root planing in the treatment of chronic periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Giannelli, M.; Lasagni, M.; Bani, D. Photonic Therapy in Periodontal Diseases an Overview with Appraisal of the Literature and Reasoned Treatment Recommendations. Int. J. Mol. Sci. 2019, 20, 4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betsy, J.; Prasanth, C.S.; Baiju, K.V.; Prasanthila, J.; Subhash, N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Dehn, C.; Krause, F.; Jepsen, S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008, 35, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Lulic, M.; Leiggener Görög, I.; Salvi, G.E.; Ramseier, C.A.; Mattheos, N.; Lang, N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 661–666. [Google Scholar] [CrossRef]
- Alwaeli, H.A.; Al-Khateeb, S.N.; Al-Sadi, A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 801–807. [Google Scholar] [CrossRef]
- Sigusch, B.W.; Engelbrecht, M.; Völpel, A.; Holletschke, A.; Pfister, W.; Schütze, J. Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. J. Periodontol. 2010, 81, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Theodoro, L.H.; Assem, N.Z.; Longo, M.; Alves, M.L.F.; Duque, C.; Stipp, R.N.; Vizoto, N.L.; Garcia, V.G. Treatment of periodontitis in smokers with multiple sessions of antimicrobial photodynamic therapy or systemic antibiotics: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018, 22, 217–222. [Google Scholar] [CrossRef]
- Harmouche, L.; Courval, A.; Mathieu, A.; Petit, C.; Huck, O.; Severac, F.; Davideau, J.-L. Impact of tooth-related factors on photodynamic therapy effectiveness during active periodontal therapy: A 6-months split-mouth randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019, 27, 167–172. [Google Scholar] [CrossRef]
- Kolakovic, M.; Held, U.; Schmidlin, P.R.; Sahrmann, P. An estimate of pocket closure and avoided needs of surgery after scaling and root planing with systemic antibiotics: A systematic review. BMC Oral Health 2014, 14, 159. [Google Scholar] [CrossRef] [Green Version]
- Cosgarea, R.; Heumann, C.; Juncar, R.; Tristiu, R.; Lascu, L.; Salvi, G.E.; Arweiler, N.B.; Sculean, A. One year results of a randomized controlled clinical study evaluating the effects of non-surgical periodontal therapy of chronic periodontitis in conjunction with three or seven days systemic administration of amoxicillin/metronidazole. PLoS ONE 2017, 12, e0179592. [Google Scholar] [CrossRef]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A. How effective is surgical therapy compared with nonsurgical debridement? Periodontology 2000 2005, 37, 72–87. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Hamp, S.E.; Nyman, S.; Lindhe, J. Periodontal treatment of multirooted teeth. Results after 5 years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, W.; Davideau, J.-L.; Tenenbaum, H.; Huck, O. Adiposity Measurements and Non-Surgical Periodontal Therapy Outcomes. J. Periodontol. 2015, 86, 1030–1037. [Google Scholar] [CrossRef]
- Bassir, S.H.; Moslemi, N.; Jamali, R.; Mashmouly, S.; Fekrazad, R.; Chiniforush, N.; Shamshiri, A.R.; Nowzari, H. Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: A pilot double-blind split-mouth randomized clinical trial. J. Clin. Periodontol. 2013, 40, 65–72. [Google Scholar] [CrossRef]
- Azaripour, A.; Dittrich, S.; Van Noorden, C.J.F.; Willershausen, B. Efficacy of photodynamic therapy as adjunct treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2018, 33, 407–423. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.-L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef]
- Tabenski, L.; Moder, D.; Cieplik, F.; Schenke, F.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Christgau, M. Antimicrobial photodynamic therapy vs. local minocycline in addition to non-surgical therapy of deep periodontal pockets: A controlled randomized clinical trial. Clin. Oral Investig. 2017, 21, 2253–2264. [Google Scholar] [CrossRef]
- Claffey, N.; Polyzois, I.; Ziaka, P. An overview of nonsurgical and surgical therapy. Periodontology 2000 2004, 36, 35–44. [Google Scholar] [CrossRef]
- Goh, E.X.; Tan, K.S.; Chan, Y.H.; Lim, L.P. Effects of root debridement and adjunctive photodynamic therapy in residual pockets of patients on supportive periodontal therapy: A randomized split-mouth trial. Photodiagnosis Photodyn. Ther. 2017, 18, 342–348. [Google Scholar] [CrossRef]
- Queiroz, L.A.; Casarin, R.C.V.; Dabdoub, S.M.; Tatakis, D.N.; Sallum, E.A.; Kumar, P.S. Furcation Therapy with Enamel Matrix Derivative: Effects on the Subgingival Microbiome. J. Periodontol. 2017, 88, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.; Claffey, N.; Egelberg, J. Clinical and microbiological effects of root debridement in periodontal furcation pockets. J. Clin. Periodontol. 1988, 15, 453–463. [Google Scholar] [CrossRef]
- Müller Campanile, V.S.; Giannopoulou, C.; Campanile, G.; Cancela, J.A.; Mombelli, A. Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: Clinical, microbiological, and local biological effects. Lasers Med. Sci. 2015, 30, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Hayashi, J.-I.; Fujimura, T.; Iwamura, Y.; Yamamoto, G.; Nishida, E.; Ohno, T.; Okada, K.; Yamamoto, H.; Kikuchi, T.; et al. New Irradiation Method with Indocyanine Green-Loaded Nanospheres for Inactivating Periodontal Pathogens. Int. J. Mol. Sci. 2017, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Sun, C.; Akcalı, A.; Yeh, Y.-C.; Tu, Y.-K.; Donos, N. The effect of horizontal and vertical furcation involvement on molar survival: A retrospective study. J. Clin. Periodontol. 2018, 45, 373–381. [Google Scholar] [CrossRef] [PubMed]
| N = 36 | |
|---|---|
| Age (years) | 50.25 ± 5.98 |
| Women, n (%) | 22 (61.1) |
| Smoker n (%) | 11 (30.5) |
| Molar Furcations | Other Sites | |||
|---|---|---|---|---|
| SRP + PDT | SRP | SRP + PDT | SRP | |
| PPD > 5 mm nb (%) | ||||
| Baseline | 70(21.34) | 87(26.77) | 509(20.47) | 522(21.07) |
| 3-months | 42(14.09) | 37(12.80) | 165(7.31) | 236(10.41) |
| 6-months | 26(10.48) | 29(11.33) | 115(6.01) | 142(7.39) |
| BOP nb (%) | ||||
| Baseline | 215(64.76) | 207(64.89) | 1597(64.60) | 1615(64.91) |
| 3-months | 111(37.50) | 128(43.84) | 715(31.72) | 764(33.78) |
| 6-months | 107(43.32) | 107(41.80) | 594(31.08) | 616(31.97) |
| Mean PPD mm (SD) | ||||
| Baseline | 4.28(1.77) | 4.32(1.72) | 4.02(1.70) | 4.06(1.71) |
| 3-months | 3.68(1.84) | 3.71(1.61) | 3.12(1.54) | 3.23(1.58) |
| 6-months | 3.37(1.57) | 3.44(1.51) | 2.87(1.40) | 2.87(1.38) |
| Mean CAL mm (SD) | ||||
| Baseline | 5.18(2.14) | 5.18(2.16) | 4.73(2.05) | 4.71(2.04) |
| 3-months | 4.68(2.30) | 4.73(2.12) | 4.03(1.97) | 4.06(1.97) |
| 6-months | 4.52(2.18) | 4.60(2.09) | 3.86(1.95) | 3.82(1.88) |
| PI > 1 nb (%) | ||||
| Baseline | 55(16.65) | 64(19.81) | 551(22.23) | 557(22.37) |
| 3-months | 58(19.66) | 57(19.59) | 337(14.99) | 308(13.67) |
| 6-months | 29(13.28) | 34(13.28) | 213(11.09) | 242(12.66) |
| Molar Furcations | ||
|---|---|---|
| SRP + PDT | SRP | |
| PPD > 5 mm nb (%) | 70(21.3) | 87(26.8) |
| PPD > 6 mm nb (%) | 34(10.4) | 38(11.7) |
| Cl 0–I nb (%) | 186(81.2) | 176(76.7) |
| Cl II–III nb (%) | 43(18.7) | 55(23.8) |
| PPD > 5 mm Cl 0–I nb (%) | 30(16.1) | 43(24.4) |
| PPD > 5 mm Cl II–III nb (%) | 17(39.9) | 24(43.6) |
| Molar Furcations | Other Sites | |||||
|---|---|---|---|---|---|---|
| OR/MR | CI | p-value | OR/MR | CI | p-value | |
| PPD > 5 mm nb (%) | ||||||
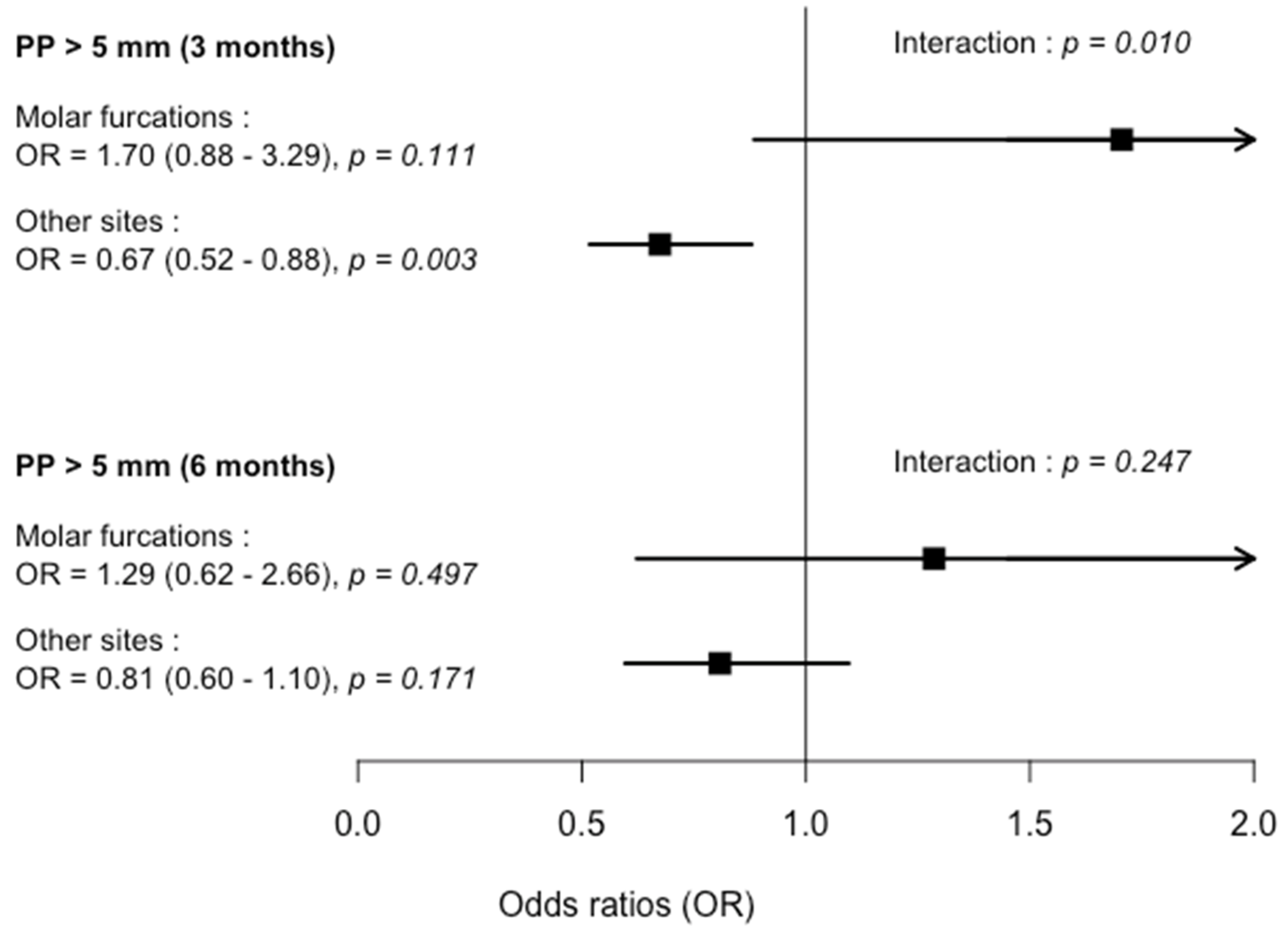
| 3-months | 1.70 | (0.884–3.289) | 0.111 | 0.67 | (0.517–0.879) | 0.003 * |
| 6-months | 1.28 | (0.622–2.658) | 0.497 | 0.80 | (0.596–1.096) | 0.171 |
| BOP nb (%) | ||||||
| 3-months | 0.70 | (0.418–1.190) | 0.191 | 0.87 | (0.722–1.053) | 0.154 |
| 6-months | 1.09 | (0.638–1.877) | 0.744 | 0.91 | (0.753–1.117) | 0.391 |
| Mean PPD mm (SD) | ||||||
| 3-months | 1.010 | (0.933–1.094) | 0.800 | 0.975 | (0.948–1.004) | 0.089 |
| 6-months | 0.993 | (0.914–1.079) | 0.871 | 1.006 | (0.976–1.036) | 0.713 |
| Mean CAL mm (SD) | ||||||
| 3-months | 0.996 | (0.912–1.088) | 0.929 | 0.984 | (0.954–1.016) | 0.331 |
| 6-months | 0.984 | (0.898–1.078) | 0.732 | 1.006 | (0.973–1.040) | 0.733 |
| PI > 1 nb (%) | ||||||
| 3-months | 1.38 | (0.729–2.632) | 0.320 | 1.15 | (0.908–1.476) | 0.237 |
| 6-months | 1.09 | (0.530–2.243) | 0.813 | 1.15 | (0.887–1.500) | 0.287 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courval, A.; Harmouche, L.; Mathieu, A.; Petit, C.; Huck, O.; Séverac, F.; Davideau, J.-L. Impact of Molar Furcations on Photodynamic Therapy Outcomes: A 6-Month Split-Mouth Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 4162. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17114162
Courval A, Harmouche L, Mathieu A, Petit C, Huck O, Séverac F, Davideau J-L. Impact of Molar Furcations on Photodynamic Therapy Outcomes: A 6-Month Split-Mouth Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2020; 17(11):4162. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17114162
Chicago/Turabian StyleCourval, Aymeric, Laetitia Harmouche, Anne Mathieu, Catherine Petit, Olivier Huck, François Séverac, and Jean-Luc Davideau. 2020. "Impact of Molar Furcations on Photodynamic Therapy Outcomes: A 6-Month Split-Mouth Randomized Clinical Trial" International Journal of Environmental Research and Public Health 17, no. 11: 4162. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17114162





