No-Touch Automated Disinfection System for Decontamination of Surfaces in Hospitals
Abstract
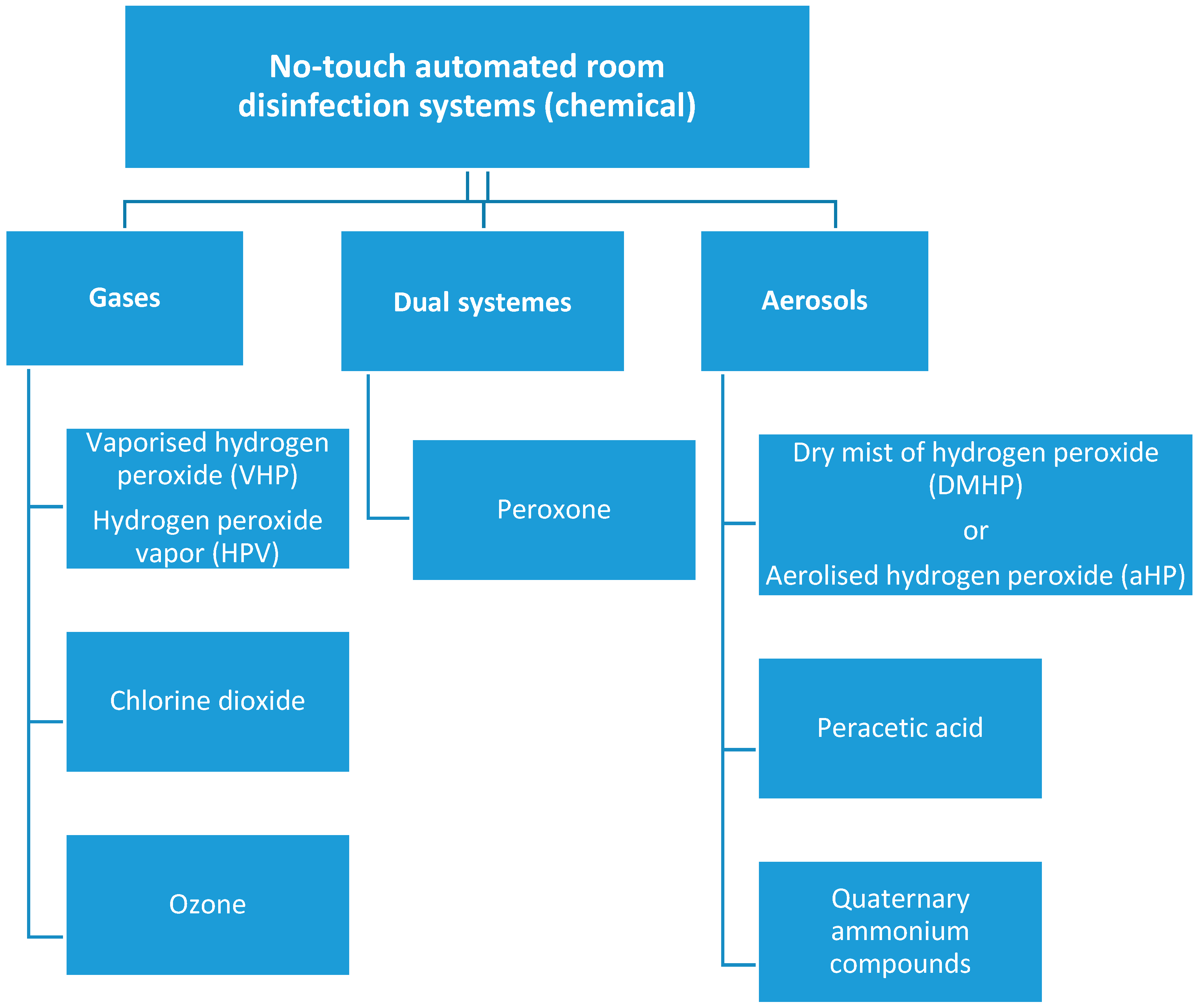
:1. Introduction
2. Material and Methods
2.1. Study Design and Sample Collection
- Disinfectant compound usage: 8 mL/m3
- Particle size generated by the device: 5–8 µm (dry mist)
- Hydrogen peroxide concentration: 7.5%
- Maximum ozone concentration: 3 ppm
- Contact time—1 h.
- Aerating time—1 h.
2.2. ATP Bioluminescence Assays
2.3. Microbiological Assay
2.4. Statistical Analysis
3. Results
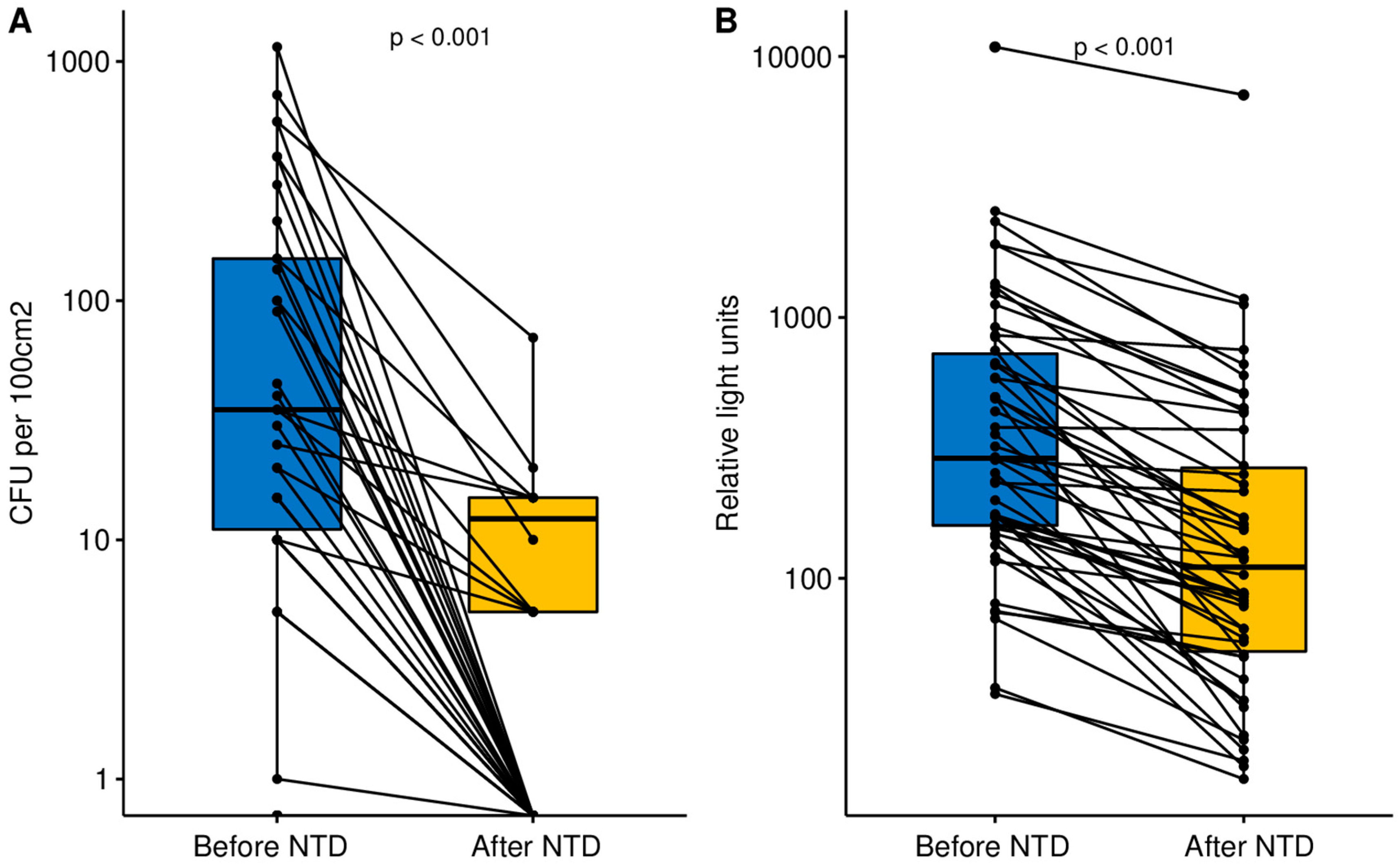
3.1. Microbiological Assay
3.2. ATP Bioluminescence Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klevens, R.M.; Edwards, J.R.; Richards, C.L. Estimating healthcare-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef]
- Anderson, R.N. Deaths: Leading causes for 1999. Natl. Vital. Stat. Rep. 2001, 49, 1–87. [Google Scholar] [PubMed]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Sin, M.A.; Blank, H.-P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R., II. The Direct Medical Costs of Healthcare-Associated Infections in US Hospitals and the Benefits of Prevention. In Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Coordinating Center for Infectious Diseases, Centers for Disease Control and Prevention; Polock, D.A., Stone, P.W., Eds.; Economist: London, UK, 2009. [Google Scholar]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Sole, M.L.; Poalillo, F.E.; Byers, J.F.; Ludy, J.E. Bacterial growth in secretions and on suctioning equipment of orally intubated patients: A pilot study. Am. J. Crit. Care 2002, 11, 141–149. [Google Scholar] [CrossRef]
- Catalano, M.; Quelle, L.S.; Jeric, P.E.; Di Martino, A.; Maimone, S.M. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J. Hosp. Infect. 1999, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Heyba, M.; Ismaiel, M.; Alotaibi, A.; Mahmoud, M.; Baquer, H.; Safar, A.; Al-Sweih, N.; Al-Tailr, A. Microbiological contamination of mobile phones of clinicians in intensive care units and neonatal care units in public hospitals in Kuwait. BMC Infect. Dis. 2015, 15, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, B.; Benson, M.; Junger, A.; Quinzio, L.; Rohring, R.; Fengler, B.; Farber, U.W.; Wille, B.; Hempelmann, G. Computer keyboard and mouse as a reservoir of pathogens in an intensive care unit. J. Clin. Monit. Comput. 2004, 18, 7–12. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.F. National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.F. National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. Infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.P.R.; Smyth, D.; Moore, G.; Singleton, J.; Jackson, R.; Gant, V.; Jeanes, A.; Shaw, S.; James, E.; Cooper, B.; et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: A randomized crossover study in critical care units in two hospitals. Crit. Care Med. 2011, 39, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning Hospital Room Surfaces to Prevent Health Care-Associated Infections: A Technical Brief. Ann. Intern. Med. 2015, 20, 598–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otter, J.A.; Yezli, S.; Perl, T.M.; Barbut, F.; French, G.L. 17—A guide to no-touch automated room disinfection (NTD) systems. In Decontamination in Hospitals and Healthcare; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 413–460. [Google Scholar]
- Casini, B.; Tuvo, B.; Cristina, M.L.; Spagnolo, A.M.; Totaro, M.; Baggiani, A.; Privitera, G.P. Evaluation of an ultraviolet C (UVC) light-emitting device for disinfection of high touch surfaces in hospital critical areas. Int. J. Environ. Res. Public Health 2019, 16, 3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgujja, A.; Altalhi, H.; Ezreqat, S. Review of the efficacy of ultraviolet C for surface decontamination. J. Nat. Sci. Med. 2020, 3, 8–12. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Perl, T.M.; Barbut, F.; French, G.L. The role of “no-touch” automated room disinfection systems in infection prevention and control. J. Hosp. Infect. 2013, 83, 1–13. [Google Scholar] [CrossRef]
- Xu, X.; Goddard, W.A., III. Peroxone chemistry: Formation of H2O3 and ring-(HO2) (HO3) from O3/H2O2. Proc. Natl. Acad. Sci. USA 2002, 99, 15308–15312. [Google Scholar] [CrossRef] [Green Version]
- Moore, G.; Smyth, D.; Singleton, J.; Wilson, P. The use of adenosine triphosphate bioluminescence to assess the efficacy of a modified cleaning program implemented within an intensive care setting. Am. J. Infect. Control 2010, 38, 617–662. [Google Scholar] [CrossRef]
- Shama, G.; Malik, D. The uses and abuses of rapid bioluminescence-based ATP assays. Int. J. Hydrogen Environ. Health 2013, 216, 115–125. [Google Scholar] [CrossRef] [Green Version]
- European Committee for Standardization. CEN/TC 243—Cleanroom Technology. 1993. Available online: https://standards.iteh.ai/catalog/tc/cen/9f58539c-5394-4f44-afe3-8528a2e35e33/cen-tc-243 (accessed on 1 May 2020).
- Rawlinson, S.; Ciric, L.; Cloutman-Green, E. How to carry out microbiological sampling of healthcare environment surfaces? A review of current evidence. J. Hosp. Inf. 2019, 103, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Draft European Standard CEN/TC/243/WG2/1993. Available online: https://www.hex-group.eu/en_BE/biocontamination-control/ (accessed on 1 May 2020).
- Eschlbeck, E.; Seeburger, C.; Kulozik, U. Spore inactivation on solid surfaces by vaporized hydrogen peroxide—Influence of carrier material surface properties. J. Food. Sci. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, H.; Niemeyer, B. Aerosol disinfection of bacterial spores by peracetic acid on antibacterial surfaces and other technical materials. Am. J. Infect. Control 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chen, Y.C.; Chen, M.L.; Cheng, A.; Hung, I.C.; Wang, J.T.; Sheng, W.H.; Chang, S.C. Comparing visual inspection, aerobic colony counts, and adenosine triphosphate bioluminescence assay for evaluating surface cleanliness at a medical center. Am. J. Infect. Control. 2015, 43, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Colbert, M.E.; Gibbs, S.G.; Schmid, K.K.; High, R.; Lowe, J.J.; Chaika, O.; Smith, P.W. Evaluation of adenosine triphosphate (ATP) bioluminescence assay to confirm surface disinfection of biological indicators with vaporised hydrogen peroxide (VHP). Healthc. Infect. 2015, 20, 16–22. [Google Scholar] [CrossRef]
- Nante, N.; Ceriale, E.; Messina, G.; Lenzi, D.; Manzi, P. Effectiveness of ATP bioluminescence to assess hospital cleaning: A review. J. Prev. Med. Hyg. 2017, 58, E177–E183. [Google Scholar]
- EN 17272:2020. Chemical Disinfectants and Antiseptics—Methods of Airborne Room Disinfection by Automated Process —Determination of Bactericidal, Mycobactericidal, Sporicidal, Fungicidal, Yeasticidal, Virucidal and Phagocidal Activities. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_LANG_ID,FSP_PROJECT:25,62318&cs=10D54BE215B9679AD9914C42C71BA77CE (accessed on 1 May 2020).
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 2010, 38, S25–S33. [Google Scholar] [CrossRef]
- Datta, R.; Platt, R.; Yokoe, D.S.; Huang, S.S. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch. Intern. Med. 2011, 171, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.S.; Datta, R.; Platt, R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch. Intern. Med. 2006, 166, 1945–1951. [Google Scholar] [CrossRef] [Green Version]
- Drees, M.; Snydman, D.; Schmid, C. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin. Infect. Dis. 2008, 46, 678–685. [Google Scholar] [CrossRef]
- Nseir, S.; Blazejewski, C.; Lubret, R.; Wallet, F.; Courcol, R.; Durocher, A. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the ICU. Clin. Microbiol. Infect. 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Bshabshe, A.A.; Joseph, M.R.P.; Assiri, A.; Omer, H.A.; Hamid, M.E. A multimodality approach to decreasing ICU infections by hydrogen peroxide, silver cations, and compartmentalization. J. Infect. Public Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Rognoni, V.; Accetta, R.; Ferrari, M. Impact of hydrogen peroxide and silver cations dry-mist disinfection on the reduction of hospital acquired Clostridium difficile infections. In Proceedings of the 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018. [Google Scholar]
- Fudan Zhongshan. Guidance of COVID-19 Prevention and Control. Available online: https://www.tencent.com/en-us/responsibility/combat-covid-19-hospital-guidance.html (accessed on 1 May 2020).
- Carling, P.C.; Von Beheren, S.; Kim, P.; Woods, C. Healthcare Environmental Hygiene Study Group. Intensive care unit environmental cleaning: An evaluation in sixteen hospitals using a novel assessment tool. J. Hosp. Infect. 2008, 68, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chiguer, M.; Maleb, A.; Amrani, R.; Abda, N.; Alami, Z. Assessment of surface cleaning and disinfection in neonatal intensive care unit. Heliyon 2019, 5, E2966. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Eder, A.R.; Thompson, K.M. Do surface and cleaning chemistries interfere with ATP measurement systems for monitoring patient room hygiene? J. Hosp. Infect. 2010, 74, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Griffith, C.; Gallo, M.; Weinbren, M. A modified ATP benchmark for evaluating the cleaning of some hospital environmental surfaces. J. Hosp. Infect. 2008, 69, 156–163. [Google Scholar] [CrossRef]
- Mulvey, D.; Redding, P.; Robertson, C.; Woodall, C.; Kingsmore, P.; Bedwell, D.; Dancer, S.J. Finding a benchmark for monitoring hospital cleanliness. J. Hosp. Infect. 2011, 77, 25–30. [Google Scholar] [CrossRef]
- Smith, P.W.; Sayles, H.; Hewlett, A.; Cavalieri, R.J.; Gibbs, S.G.; Rupp, M.E. A study of three methods for assessment of hospital environmental cleaning. Healthc. Infect. 2013, 18, 80–85. [Google Scholar] [CrossRef]
- Ho, Y.H.; Wang, L.S.; Jiang, H.L.; Chang, C.H.; Hsieh, C.J.; Chang, D.C.; Tu, H.Y.; Chiu, T.Y.; Chao, H.J.; Tseng, C.C. Use of a Sampling Area-Adjusted Adenosine Triphosphate Bioluminescence Assay Based on Digital Image Quantification to Assess the Cleanliness of Hospital Surfaces. Int. J. Environ. Res. Public. Health. 2016, 13, 576. [Google Scholar] [CrossRef]
- Griffith, C.J.; Obee, P.; Cooper, R.A.; Burton, N.F.; Lewis, M. The effectiveness of existing and modified cleaning regimens in a Welsh hospital. J. Hosp. Infect. 2007, 66, 352–359. [Google Scholar] [CrossRef]
- Carling, P.C.; Perkins, J.; Ferguson, J.; Thomasser, A. Evaluating a New Paradigm for Comparing Surface Disinfection in Clinical Practice. Infect. Control. Hosp. Epidemiol. 2014, 35, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
| Microbiological Assay (CFU/100 cm2) | ATP Bioluminescence Assay (RLU) | ||||
|---|---|---|---|---|---|
| Room | Surface | Before NTD | After NTD | Before NTD | After NTD |
| Operating Room 4 | Operating lamp holder | 15 | 0 | 379 | 371 |
| Upper surface of operating table | 5 | 0 | 172 | 85 | |
| Anaesthetic machine (table) | 45 | 0 | 159 | 78 | |
| Mayo table | 0 | 0 | 172 | 64 | |
| Phone | 560 | 0 | 2331 | 599 | |
| Treatment Room in Ward I | Treatment lamp holder | 1150 | 0 | 1119 | 508 |
| Upper surface of mattress | 10 | 5 | 155 | 103 | |
| Treatment table | 150 | 0 | 840 | 120 | |
| Sink | 15 | 0 | 176 | 22 | |
| Locker holder under sink | 5 | 0 | 587 | 87 | |
| Patient Recovery Room 1 | Phone | 560 | 70 | 2552 | 1178 |
| Bed remote control | 10 | 0 | 668 | 229 | |
| Bed railing | 215 | 0 | 1905 | 1119 | |
| Ambu bag | 400 | 10 | 10,874 | 7123 | |
| Sink | 10 | 0 | 253 | 32 | |
| Endoscopy Unit: automatic disinfector room | Sink | 0 | 0 | 70 | 24 |
| Locker holder | 400 | 0 | 356 | 64 | |
| Remote control of disinfector | 0 | 0 | 746 | 51 | |
| Inside of automatic disinfector | 0 | 0 | 144 | 19 | |
| Tap above sink | 10 | 0 | 320 | 161 | |
| Endoscopy Unit: examination room | Video track endoscopy keyboard | 305 | 0 | 656 | 156 |
| Blood pressure cuff | 40 | 0 | 1308 | 270 | |
| Anaesthesiological table | 90 | 0 | 74 | 57 | |
| Storage | 35 | 5 | 436 | 171 | |
| Anaesthesiological table handle | 725 | 20 | 488 | 170 | |
| Operating Room 1 | Upper surface of operating table | 0 | 0 | 175 | 87 |
| Operating lamp holder | 0 | 0 | 75 | 50 | |
| Computer mouse | 25 | 15 | 1230 | 514 | |
| X-ray apparatus keyboard | 35 | 15 | 854 | 751 | |
| Ambu bag | 0 | 0 | 116 | 88 | |
| Operating Room 2 | Infusion pump remote control panel | 0 | 0 | 918 | 450 |
| Ambu bag | 135 | 0 | 1349 | 437 | |
| Anaesthesiological table | 0 | 0 | 148 | 82 | |
| Blood pressure cuff | 20 | 0 | 121 | 34 | |
| Operating table remote control panel | 0 | 0 | 583 | 430 | |
| Ward no. 116 | Calling staff pilot | 1 | 0 | 80 | 50 |
| Mattress | 20 | 5 | 291 | 153 | |
| Toilet flush button | 0 | 0 | 38 | 17 | |
| Bedside table | 100 | 5 | 235 | 127 | |
| Bed railing | 30 | 0 | 232 | 215 | |
| Patient Recovery Room 2 | Infusion pump control panel | 0 | 0 | 160 | 120 |
| Sink | 0 | 0 | 286 | 80 | |
| Bedside table | 0 | 0 | 495 | 118 | |
| Cardiomonitor screen | 0 | 0 | 284 | 250 | |
| Handle locker | 0 | 0 | 173 | 34 | |
| Treatment Room Ward I | Bed remote control | 5 | 0 | 495 | 25 |
| Sink | 10 | 0 | 36 | 20 | |
| ECG apparatus keyboard | 150 | 15 | 1905 | 661 | |
| Treatment lamp holder | 15 | 0 | 134 | 41 | |
| Upper surface of mattress | 100 | 0 | 199 | 59 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarka, P.; Nitsch-Osuch, A. No-Touch Automated Disinfection System for Decontamination of Surfaces in Hospitals. Int. J. Environ. Res. Public Health 2020, 17, 5131. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17145131
Tarka P, Nitsch-Osuch A. No-Touch Automated Disinfection System for Decontamination of Surfaces in Hospitals. International Journal of Environmental Research and Public Health. 2020; 17(14):5131. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17145131
Chicago/Turabian StyleTarka, Patryk, and Aneta Nitsch-Osuch. 2020. "No-Touch Automated Disinfection System for Decontamination of Surfaces in Hospitals" International Journal of Environmental Research and Public Health 17, no. 14: 5131. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17145131





