Synthetic Bone Grafting in Aseptic Loosening of Acetabular Cup: Good Clinical and Radiological Outcomes in Contained Bone Defects at Medium-Term Follow Up
Abstract
:1. Introduction
2. Materials and Methods
Ethical Statement
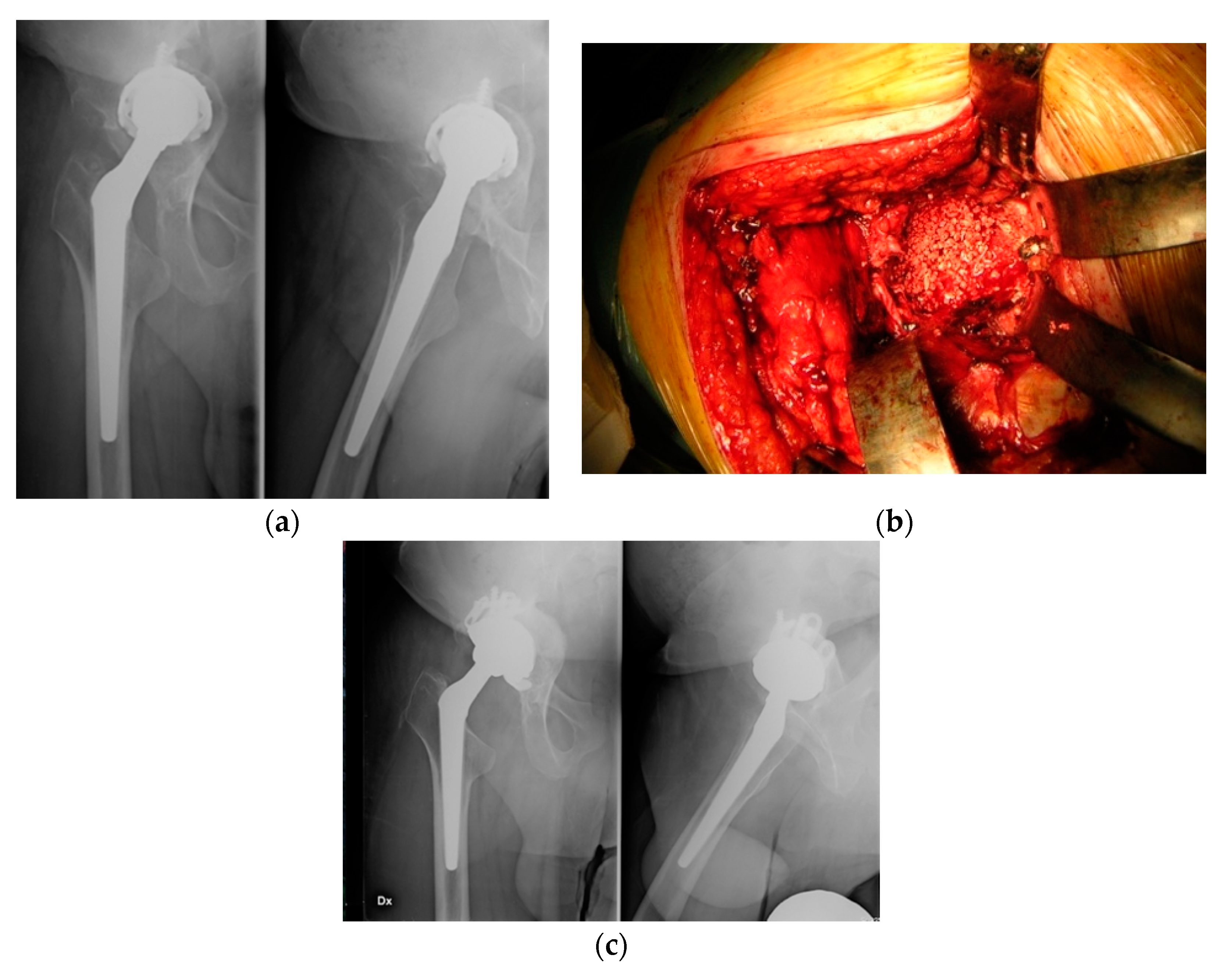
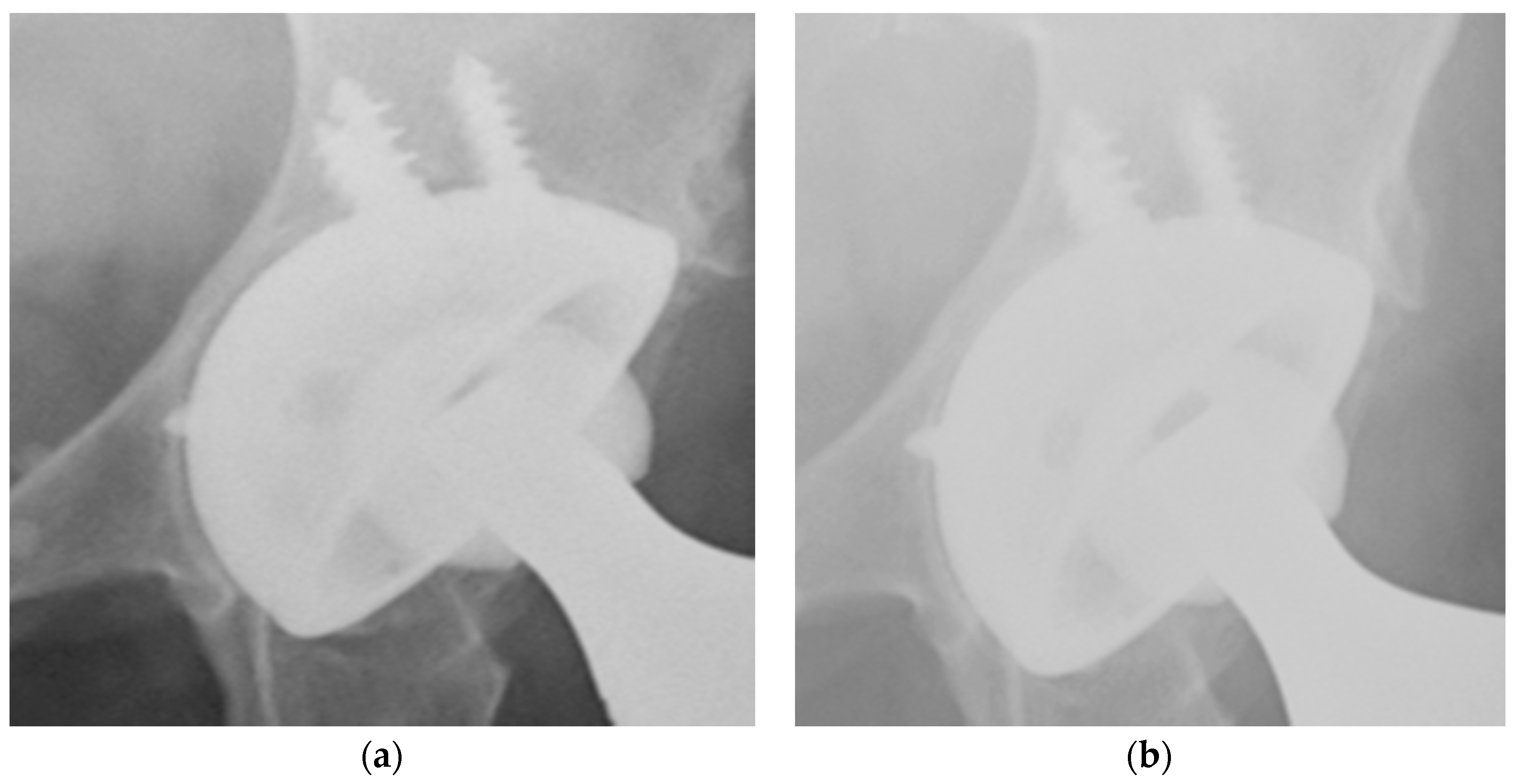
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gwam, C.U.; Mistry, J.B.; Mohamed, N.S.; Thomas, M.; Bigart, K.C.; Mont, M.A.; Delanois, R.E. Current epidemiology of revision total hip arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J. Arthroplast. 2017, 32, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. JBJS 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, T.P.; Berry, D.J. The epidemiology of revision total hip arthroplasty in the United States. JBJS 2009, 91, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.W.; Wylde, V.; Livesey, C.; Whitehouse, M.R.; Eastaugh-Waring, S.; Bannister, G.C.; Learmonth, I.D. Impaction bone grafting of the acetabulum at hip revision using a mix of bone chips and a biphasic porous ceramic bone graft substitute: Good outcome in 43 patients followed for a mean of 2 years. Acta Orthop. 2009, 80, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hastings, D.E.; Parker, S.M. Protrusio acetabuli in rheumatoid arthritis. Clin. Orthop. Relat. Res. 1975, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Slooff, T.J.J.H.; Huiskes, R.; van Horn, J.; Lemmens, A.J. Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop. Scand. 1984, 55, 593–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulido, L.; Rachala, S.R.; Cabanela, M.E. Cementless acetabular revision: Past, present, and future. Int. Orthop. 2011, 35, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, M.R.; Dacombe, P.J.; Webb, J.C.J.; Blom, A.W. Impaction grafting of the acetabulum with ceramic bone graft substitute mixed with femoral head allograft: High survivorship in 43 patients with a median follow-up of 7 years. Acta Orthop. 2013, 84, 365–370. [Google Scholar] [CrossRef]
- Palm, L.; Jacobsson, S.-A.; Kvist, J.; Lindholm, A.; Öjersjö, A.; Ivarsson, I. Acetabular revision with extensive allograft impaction and uncemented hydroxyapatite-coated implants. Results after 9 (7-11) years follow-up. J. Arthroplast. 2007, 22, 1083–1091. [Google Scholar] [CrossRef]
- Pierannunzii, L.; Zagra, L. Bone grafts, bone graft extenders, substitutes and enhancers for acetabular reconstruction in revision total hip arthroplasty. EFORT Open Rev. 2016, 1, 431–439. [Google Scholar] [CrossRef]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98 (Suppl. A), 6–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Sollazzo, V.; Palmieri, A.; Girardi, A.; Farinella, F.; Carinci, F. Engipore acts on human bone marrow stem cells. Saudi Dent. J. 2010, 22, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Sheth, N.P.; Nelson, C.L.; Springer, B.D.; Fehring, T.K.; Paprosky, W.G. Acetabular bone loss in revision total hip arthroplasty: Evaluation and management. JAAOS J. Am. Acad. Orthop. Surg. 2013, 21, 128–139. [Google Scholar] [CrossRef]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.K.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar] [PubMed]
- Söderman, P.; Malchau, H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin. Orthop. Relat. Res. 2001, 384, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Söderman, P.; Malchau, H. Validity and reliability of Swedish WOMAC osteoarthritis index: A self-administered disease-specific questionnaire (WOMAC) versus generic instruments (SF-36 and NHP). Acta Orthop. Scand. 2000, 71, 39–46. [Google Scholar] [CrossRef]
- Banaszkiewicz, P.A. Radiological demarcation of cemented sockets in total hip replacement. In Classic Papers in Orthopaedics; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–41. [Google Scholar]
- Brooker, A.F.; Bowerman, J.W.; Robinson, R.A.; Riley, L.H., Jr. Ectopic ossification following total hip replacement: Incidence and a method of classification. JBJS 1973, 55, 1629–1632. [Google Scholar] [CrossRef]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Jt. J. 2013, 95, 583–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sporer, S.M.; Paprosky, W.G.; O’Rourke, M.R. Managing bone loss in acetabular revision. Instr. Course Lect. 2006, 55, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.S.; Raja, S.; Haddad, F.S. Acetabular impaction bone grafting in total hip replacement. Bone Jt. J. 2013, 95 (Suppl. A), 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oonishi, H.; Iwaki, Y.; Kin, N.; Kushitani, S.; Murata, N.; Wakitani, S.; Imoto, K. Hydroxyapatite In Revision Of Total Hip Replacements With Massive Acetabular Defects: 4- To 10-year Clinical Results. J. Bone Jt. Surg. Br. 1997, 79, 87–92. [Google Scholar] [CrossRef]
- Schwartz, C.; Vautrin, M. Phosphocalcium ceramics are efficient in the management of severe acetabular loss in revision hip arthroplasties. A 22 cases long-term follow-up study. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 227–232. [Google Scholar] [CrossRef]
- Wasielewski, R.; Sheridan, K.; Lubbers, M. Coralline Hydroxyapatite in Complex Acetabular Reconstruction. Orthopedics 2008, 31, 367. [Google Scholar] [CrossRef]
- Kumar, V.; Ricks, M.; Abouel-Enin, S.; Dunlop, D.G. Long term results of impaction Bone grafting using a synthetic graft (Apapore) in revision hip surgery. J. Orthop. 2013, 14, 290–293. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Jasty, M.; Harris, W.H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J. Bone Jt. Surg. Am. 1992, 74, 849–863. [Google Scholar] [CrossRef]
- McNamara, I.; Deshpande, S.; Porteous, M. Impaction grafting of the acetabulum with a mixture of frozen, ground irradiated bone graft and porous synthetic bone substitute (Apapore 60). J. Bone Jt. Surg. Br. 2010, 92, 617–623. [Google Scholar] [CrossRef]
| Type | Description | Patients | Mean Age |
|---|---|---|---|
| 1 | Minimal deformity, intact rim | - | - |
| 2a | Superior bone lysis with intact superior rim | 10 | 69 years |
| 2b | Absent superior rim, superolateral migration | 8 | 70 years |
| 2c | Localized destruction of medial wall | 11 | 73 years |
| 3a | Bone loss from 10 am to 2 pm around rim with 30–60% of the supporting bone stock destroyed. There is superolateral cup migration | 7 | 76 years |
| 3b | Bone loss from 9 am to 5 pm around rim with up to 60% of the supporting bone stock destroyed. There is superomedial cup migration | - | - |
| 4 | Pelvic discontinuity | - | - |
| Clinical and Radiological Results | No. |
|---|---|
| Mean pre-operative WS | 49.7 (39–57) |
| Mean post-operative WS | 67.3 (53–81) |
| Mean pre-operative HHS | 56.1 (41–75) |
| Mean post-operative HHS | 89.4 (62–91) |
| Radiolucent lines at the last follow-up | 7 pz |
| Migration >3 mm (radiological failure) | 5 |
| Clinical failure | 1 |
| Eterotopic ossifications | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parchi, P.D.; Simonetti, M.; Bonicoli, E.; Piolanti, N.; Scaglione, M. Synthetic Bone Grafting in Aseptic Loosening of Acetabular Cup: Good Clinical and Radiological Outcomes in Contained Bone Defects at Medium-Term Follow Up. Int. J. Environ. Res. Public Health 2020, 17, 5624. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17155624
Parchi PD, Simonetti M, Bonicoli E, Piolanti N, Scaglione M. Synthetic Bone Grafting in Aseptic Loosening of Acetabular Cup: Good Clinical and Radiological Outcomes in Contained Bone Defects at Medium-Term Follow Up. International Journal of Environmental Research and Public Health. 2020; 17(15):5624. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17155624
Chicago/Turabian StyleParchi, Paolo Domenico, Matteo Simonetti, Enrico Bonicoli, Nicola Piolanti, and Michelangelo Scaglione. 2020. "Synthetic Bone Grafting in Aseptic Loosening of Acetabular Cup: Good Clinical and Radiological Outcomes in Contained Bone Defects at Medium-Term Follow Up" International Journal of Environmental Research and Public Health 17, no. 15: 5624. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17155624





