Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor
Abstract
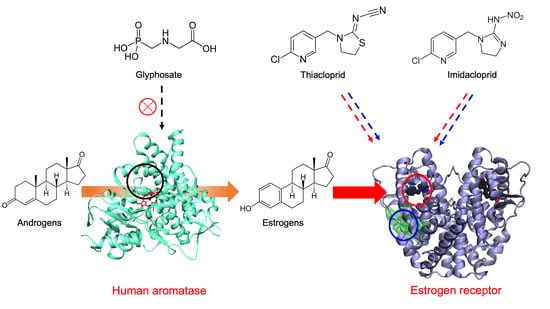
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ELISA Assay
2.3. Computational Studies
2.4. MELN cell Culture
2.5. MELN Gene Reporter Assay
2.6. Data Analysis
3. Results
3.1. Effect of Pesticides on Aromatase Activity
3.2. Effect of Glyphosate Concentration on Aromatase Activity
3.3. Effect of Glyphosate on the Catalytic Parameters of Aro
3.4. Molecular Dynamics Simulations on Aromatase
3.5. Detection of Estrogenic Activity with the MELN Gene Reporter Assay
3.6. Docking Calculation on ERα
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Kortenkamp, A.; Faust, M.; Scholze, M.; Backhaus, T. Low-level exposure to multiple chemicals: Reason for human health concerns? Environ. Health Perspect. 2007, 115, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Park, M.S.; Lee, H.S. Endocrine disrupting chemicals: Human exposure and health Risks. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2006, 24, 183–224. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; Vom, S.F.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Storck, V.; Karpouzas, D.G.; Martin-Laurent, F. Towards a better pesticide policy for the European Union. Sci. Total Environ. 2017, 575, 1027–1033. [Google Scholar] [CrossRef]
- Amrhein, N.; Deus, B.; Gehrke, P.; Steinrücken, H.C. The Site of the Inhibition of the Shikimate Pathway by glyphosate: ii. interference of glyphosate with chorismate formation in vivo and in vitro. Plant Physiol. 1980, 66, 830–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, K.M.; Weaver, L.M. The Shikimate Pathway. Annu. Rev. Plant Phys. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Tennekes, H.A. Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments. Int. J. Environ. Res. Public Health 2020, 17, 1629. [Google Scholar] [CrossRef] [Green Version]
- Mensah, P.K.; Palmer, C.G.; Odume, O.N. Ecotoxicology of glyphosate and glyphosate-based herbicides — toxicity to wildlife and humans. In Toxicity and Hazard of Agrochemicals; Larramendy, M.L., Soloneski, S., Eds.; Intech: Rijeka, Croatia, 2015; pp. 281–304. [Google Scholar]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Processes Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- The European Commission. Legislation 132. Off. J. Eur. Union 2018, 61, 31–40. [Google Scholar]
- Zhang, Q.; Li, Z.; Chang, C.H.; Lou, J.L.; Zhao, M.R.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment: Impact of glyphosate-based herbicides. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Lu, C.; Chang, C.-H.; Palmer, C.; Zhao, M.; Zhang, Q. Neonicotinoid residues in fruits and vegetables: An integrated dietary exposure assessment approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Vencill, W.K. Herbicide Handbook, 8th ed.; Vencill, W.K., Ed.; Weed Science Society of America: Lawrence, KS, USA, 2002; pp. 231–234. [Google Scholar]
- Agostini, L.P.; Dettogni, R.S.; dos Reis, R.S.; Stur, E.; dos Santos, E.V.W.; Ventorim, D.P.; Garcia, F.M.; Cardoso, R.C.; Graceli, J.B.; Louro, I.D. Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies. Sci. Total Environ. 2020, 705, 135808. [Google Scholar] [CrossRef]
- Di Nardo, G.; Gilardi, G. Human aromatase: Perspectives in biochemistry and biotechnology: Human Aromatase. Biotechnol. Appl. Biochem. 2013, 60, 92–101. [Google Scholar] [CrossRef]
- Benachour, N.; Sipahutar, H.; Moslemi, S.; Gasnier, C.; Travert, C.; Séralini, G.E. Time- and dose-dependent effects of roundup on human embryonic and placental Cells. Arch. Environ. Contam. Toxicol. 2007, 53, 126–133. [Google Scholar] [CrossRef]
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Seralini, G.-E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benachour, N.; Séralini, G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Séralini, G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Caron-Beaudoin, E.; Viau, R.; Hudon-Thibeault, A.A.; Vaillancourt, C.; Sanderson, J.T. The use of a unique co-culture model of fetoplacental steroidogenesis as a screening tool for endocrine disruptors: The effects of neonicotinoids on aromatase activity and hormone production. Toxicol. Appl. Pharmacol. 2017, 332, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Caron-Beaudoin, É.; Denison, M.S.; Sanderson, J.T. Effects of neonicotinoids on promoter-specific expression and activity of aromatase (CYP19) in human adrenocortical carcinoma (H295R) and primary umbilical vein endothelial (HUVEC) cells. Toxicol. Sci. 2016, 149, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Caron-Beaudoin, É.; Viau, R.; Sanderson, J.T. Effects of neonicotinoid pesticides on promoter-specific aromatase (CYP19) expression in Hs578t breast cancer cells and the role of the VEGF pathway. Environ. Health Perspect. 2018, 126, 047014. [Google Scholar] [CrossRef]
- Di Nardo, G.; Breitner, M.; Sadeghi, S.J.; Castrignanò, S.; Mei, G.; Di Venere, A.; Nicolai, E.; Allegra, P.; Gilardi, G. Dynamics and flexibility of human aromatase probed by FTIR and time resolved fluorescence spectroscopy. PLoS ONE 2013, 8, e82118. [Google Scholar] [CrossRef] [Green Version]
- Baravalle, R.; Ciaramella, A.; Baj, F.; Di Nardo, G.; Gilardi, G. Identification of endocrine disrupting chemicals acting on human aromatase. Biochim. Biophys. Acta - Proteins Proteomics 2018, 1866, 88–96. [Google Scholar] [CrossRef]
- Lo, J.; Di Nardo, G.; Griswold, J.; Egbuta, C.; Jiang, W.; Gilardi, G.; Ghosh, D. Structural basis for the functional roles of critical residues in human cytochrome P450 aromatase. Biochemistry 2013, 52, 5821–5829. [Google Scholar]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef]
- Pavlin, M.; Spinello, A.; Pennati, M.; Zaffaroni, N.; Gobbi, S.; Bisi, A.; Colombo, G.; Magistrato, A. A computational assay of estrogen receptor α antagonists reveals the key common structural traits of drugs effectively fighting refractory breast Cancers. Sci. Rep. 2018, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Kojima, H.; Katsura, E.; Takeuchi, S.; Niiyama, K.; Kobayashi, K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ. Health Perspect. 2004, 112, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, R.; Biserni, M.; Genkova, D.; Wesolowski, L.; Antoniou, M.N. Evaluation of neonicotinoid insecticides for oestrogenic, thyroidogenic and adipogenic activity reveals imidacloprid causes lipid accumulation. J. Appl. Toxicol. 2018, 38, 1483–1491. [Google Scholar] [CrossRef]
- Westlund, P.; Yargeau, V. Investigation of the presence and endocrine activities of pesticides found in wastewater effluent using yeast-based bioassays. Sci. Total Environ. 2017, 607–608, 744–751. [Google Scholar] [CrossRef]
- Huang, N.; Pandey, A.V.; Agrawal, V.; Reardon, W.; Lapunzina, P.D.; Mowat, D.; Jabs, E.W.; Vliet, G.V.; Sack, J.; Flück, C.E.; et al. Diversity and Function of Mutations in P450 Oxidoreductase in Patients with Antley-Bixler Syndrome and Disordered Steroidogenesis. Am. J. Hum. Genet. 2005, 76, 729–749. [Google Scholar] [CrossRef] [Green Version]
- Sgrignani, J.; Bon, M.; Colombo, G.; Magistrato, A. Computational approaches elucidate the allosteric mechanism of human aromatase inhibition: A novel possible route to small-molecule regulation of CYP450s activities? J. Chem. Inf. Model. 2014, 54, 2856–2868. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision Glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar]
- Gangloff, M.; Ruff, M.; Eiler, S.; Duclaud, S.; Wurtz, J.M.; Moras, D. Crystal structure of a mutant hERα ligand-binding domain reveals key structural features for the mechanism of partial agonism. J. Biol. Chem. 2001, 276, 15059–15065. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Spinello, A.; Martini, S.; Berti, F.; Pennati, M.; Pavlin, M.; Sgrignani, J.; Grazioso, G.; Colombo, G.; Zaffaroni, N.; Magistrato, A. Rational design of allosteric modulators of the aromatase enzyme: An unprecedented therapeutic strategy to fight breast cancer. Eur. J. Med. Chem. 2019, 168, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A.V. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucl. Aci. Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef] [Green Version]
- Spinello, A.; Pavlin, M.; Casalino, L.; Magistrato, A. A dehydrogenase dual hydrogen abstraction mechanism promotes estrogen biosynthesis: Can we expand the functional annotation of the aromatase enzyme? Chem. Eur. J. 2018, 24, 10840–10849. [Google Scholar] [CrossRef]
- Liu, B.; Dong, L.; Yu, Q.; Li, X.; Wu, F.; Tan, Z.; Luo, S. Thermodynamic study on the protonation reactions of glyphosate in aqueous solution: Potentiometry, calorimetry and NMR spectroscopy. J. Phys. Chem. B 2016, 120, 2132–2137. [Google Scholar] [CrossRef]
- Wickstrom, L.; Okur, A.; Simmerling, C. Evaluating the performance of the ff99SB force field based on NMR scalar coupling data. Biophys. J. 2009, 97, 853–856. [Google Scholar] [CrossRef] [Green Version]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Dickson, C.J.; Madej, B.D.; Skjevik, Å.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef]
- Shahrokh, K.; Orendt, A.; Yost, G.S.; Cheatham, T.E. Quantum mechanically derived AMBER-compatible heme parameters for various states of the cytochrome P450 catalytic cycle. J. Comput. Chem. 2012, 33, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graphics Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE - Antechamber Python Parser Interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N-log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Balaguer, P.; Boussioux, A.-M.; Demirpence, E.; Nicolas, J.C. Reporter cell lines are useful tools for monitoring biological activity of nuclear receptor ligands. Luminescence 2001, 16, 153–158. [Google Scholar] [CrossRef]
- Berckmans, P.; Leppens, H.; Vangenechten, C.; Witters, H. Screening of endocrine disrupting chemicals with MELN cells, an ER-transactivation assay combined with cytotoxicity assessment. Toxicol. In Vitro 2007, 21, 1262–1267. [Google Scholar] [CrossRef]
- Balaguer, P.; François, F.; Comunale, F.; Fenet, H.; Boussioux, A.-M.; Pons, M.; Nicolas, J.C.; Casellas, C. Reporter cell lines to study the estrogenic effects of xenoestrogens. Sci. Total. Environ. 1999, 233, 47–56. [Google Scholar] [CrossRef]
- Schilirò, T.; Porfido, A.; Longo, A.; Coluccia, S.; Gilli, G. The E-screen test and the MELN gene-reporter assay used for determination of estrogenic activity in fruits and vegetables in relation to pesticide residues. Food Chem. Toxicol. 2013, 62, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, W.; Hanf, V.; Schuller, W.; Kempter, C.; Metzger, J.; Hagenmaier, H. Development of a sensitive E-screen assay for quantitative analysis of estrogenic activity in municipal sewage plant effluents. Sci. Total Environ. 1999, 225, 33–48. [Google Scholar] [CrossRef]
- Gillezeau, C.; Van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magistrato, A.; Sgrignani, J.; Krause, R.; Cavalli, A. Single or multiple access channels to the CYP450s active Site? An answer from free energy simulations of the human aromatase enzyme. J. Phys. Chem. Lett. 2017, 8, 2036–2042. [Google Scholar] [CrossRef]
- Ritacco, I.; Saltalamacchia, A.; Spinello, A.; Ippoliti, E.; Magistrato, A. All-atom simulations disclose how cytochrome reductase reshapes the substrate access/egress routes of its partner CYP450s. J. Phys. Chem. Lett. 2020, 11, 1189–1193. [Google Scholar] [CrossRef]
- Scholfield, M.R.; Vander Zanden, C.M.; Carter, M.; Ho, P.S. Halogen bonding (X-bonding): A biological perspective: Halogen Bonding (X-Bonding). Protein Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef]
- Parween, S.; Di Nardo, G.; Baj, F.; Zhang, C.; Gilardi, G.; Pandey, A.V. Differential effects of variations in human P450 oxidoreductase on the aromatase activity of CYP19A1 polymorphisms R264C and R264H. J. Steroid Biochem. Mol. Biol. 2020, 196, 105507. [Google Scholar] [CrossRef]
- Zhang, C.; Catucci, G.; Di Nardo, G.; Gilardi, G. Effector role of cytochrome P450 reductase for androstenedione binding to human aromatase. Int. J. Biol. Macromol. 2020, 164, 510–517. [Google Scholar] [CrossRef]
- Cassault-Meyer, E.; Gress, S.; Séralini, G.-É.; Galeraud-Denis, I. An acute exposure to glyphosate-based herbicide alters aromatase levels in testis and sperm nuclear quality. Environ. Toxicol. Pharmacol. 2014, 38, 131–140. [Google Scholar] [CrossRef]
- Bureik, M.; Brück, N.; Hübel, K.; Bernhardt, R. The human mineralocorticoid receptor only partially differentiates between different ligands after expression in fission yeast. FEMS Yeast Res. 2005, 5, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, T.; Liu, F.; Zhang, J.; Wu, Y.; Sun, H. Occurrence and profile characteristics of the pesticide imidacloprid, preservative parabens, and their metabolites in human urine from rural and urban China. Environ. Sci. Technol. 2015, 49, 14633–14640. [Google Scholar] [CrossRef] [PubMed]
- Tapparo, A.; Giorio, C.; Marzaro, M.; Marton, D.; Soldà, L.; Girolami, V. Rapid analysis of neonicotinoid insecticides in guttation drops of corn seedlings obtained from coated seeds. J. Environ. Monit. 2011, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Defarge, N.; Takács, E.; Lozano, V.; Mesnage, R.; Spiroux de Vendômois, J.; Séralini, G.E.; Székács, A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health 2016, 13, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Pesticide | Relative Activity (%) | |||
|---|---|---|---|---|
| 500 nM | 1000 nM | 5000 nM | ||
| 1 | Glyphosate | 76.6 ± 11.3 * | 74.5 ± 7.6 * | 36.0 ± 19.5 * |
| 2 | Imidacloprid | 100.1 ± 8.8 | 92.9 ± 28.0 | 120.5 ± 17.6 |
| 3 | Thiacloprid | 100.9 ± 11.9 | 153.6 ± 56.9 | 146.9 ± 42.1 |
| Glyphosate (nM) | KM (nM) | Vmax (min−1) |
|---|---|---|
| 0 | 41.3 ± 8.2 | 0.018 ± 0.001 |
| 1000 | 35.3 ± 3.5 | 0.013 ± 0.001 * |
| 5000 | 92.3 ± 20.7 * | 0.011 ± 0.002 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Schilirò, T.; Gea, M.; Bianchi, S.; Spinello, A.; Magistrato, A.; Gilardi, G.; Di Nardo, G. Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor. Int. J. Environ. Res. Public Health 2020, 17, 5664. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17165664
Zhang C, Schilirò T, Gea M, Bianchi S, Spinello A, Magistrato A, Gilardi G, Di Nardo G. Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor. International Journal of Environmental Research and Public Health. 2020; 17(16):5664. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17165664
Chicago/Turabian StyleZhang, Chao, Tiziana Schilirò, Marta Gea, Silvia Bianchi, Angelo Spinello, Alessandra Magistrato, Gianfranco Gilardi, and Giovanna Di Nardo. 2020. "Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor" International Journal of Environmental Research and Public Health 17, no. 16: 5664. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17165664