Polycycl. Aromatic Hydrocarbon Exposure of Children in Typical Household Coal Combustion Environments: Seasonal Variations, Sources, and Carcinogenic Risks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. PAH Analysis and Quality Control
2.3. Monte Carlo Simulation
3. Results and Discussion
3.1. Seasonal Variation of Individual PAHs and PAH Congeners
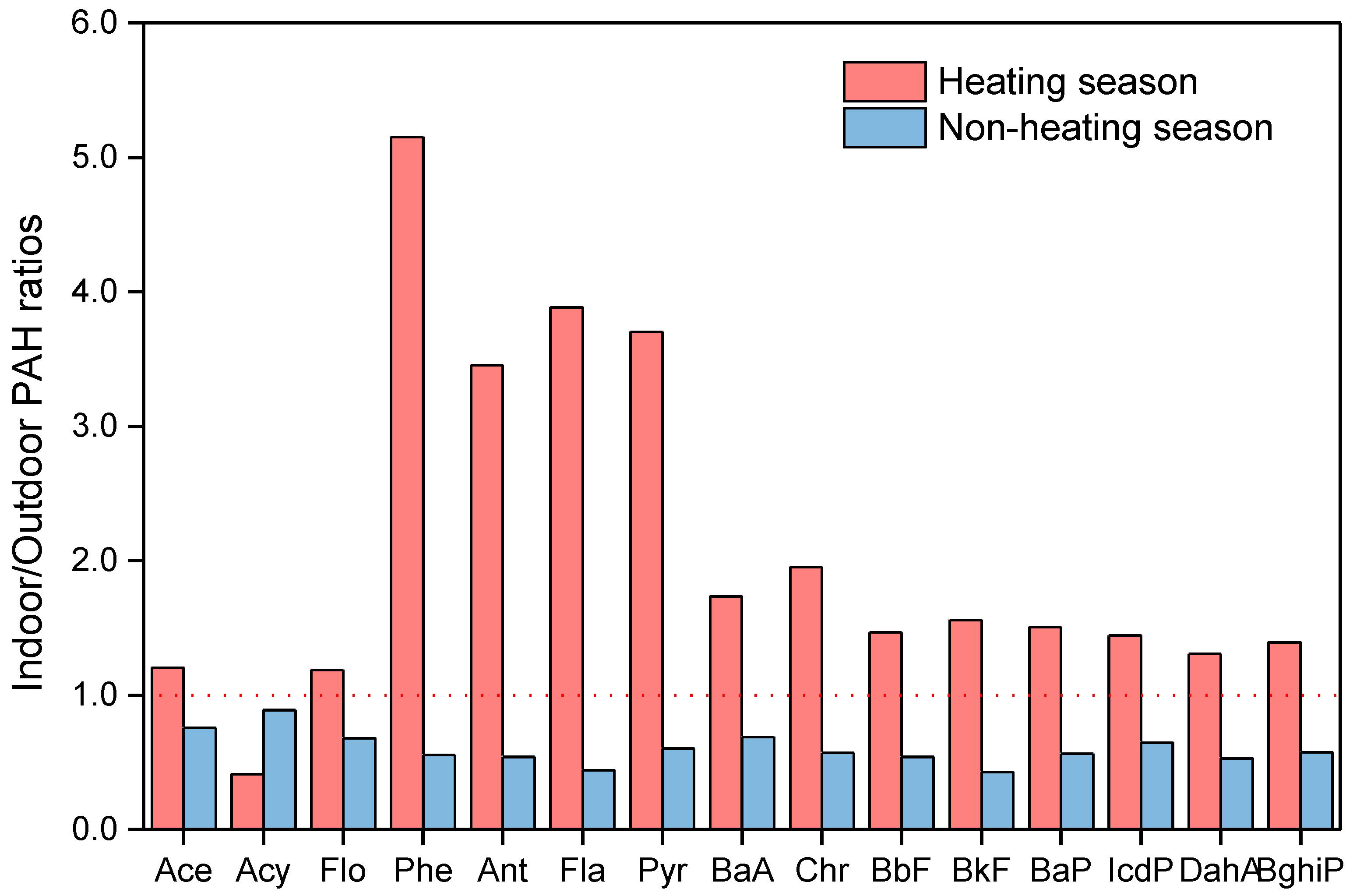
3.2. Indoor-To-Outdoor Ratios
3.3. Source Allocation
3.4. Inhalation Exposure Dose
3.5. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, B.; Liu, J. Estimating coal production peak and trends of coal imports in China. Energy Policy 2010, 38, 512–519. [Google Scholar] [CrossRef]
- Du, W.; Chen, Y.; Zhu, X.; Zhong, Q.; Zhuo, S.; Liu, W.; Huang, Y.; Shen, G.; Tao, S. Wintertime air pollution and health risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons in rural China. Atmos. Environ. 2018, 191, 1–8. [Google Scholar] [CrossRef]
- Grzegorz, W.; Patrycja, Ł.; Olga, N. Emission of some pollutants from biomass combustion in comparison to hard coal combustion. J. Energy Inst. 2017, 90, 787–796. [Google Scholar]
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.-B.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Burtscher, H.; Schüepp, K. The occurrence of ultrafine particles in the specific environment of children. Paediatr. Respir. Rev. 2012, 13, 89–94. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Guinot, B.; Li, L.; Ho, S.S.H.; Liu, S.; Li, X.; Cao, J.J.A.R. Concentrations, sources and health effects of parent, oxygenated- and nitrated- polycyclic aromatic hydrocarbons (PAHs) in middle-school air in Xi’an, China. Atmos. Res. 2017, 192, 1–10. [Google Scholar] [CrossRef]
- Salvi, S. Health effects of ambient air pollution in children. Paediatr. Respir. Rev. 2007, 8, 275–280. [Google Scholar] [CrossRef]
- Dorizas, P.V.; Assimakopoulos, M.N.; Santamouris, M. A holistic approach for the assessment of the indoor environmental quality, student productivity, and energy consumption in primary schools. Environ. Monit. Assess. Int. J. 2015, 187, 241–259. [Google Scholar]
- Tran, D.T.; Alleman, L.Y.; Coddeville, P.; Galloo, J.C. Elemental characterization and source identification of size resolved atmospheric particles in French classrooms. Atmos. Environ. 2012, 54, 250–259. [Google Scholar] [CrossRef]
- Roy, A.; Chapman, R.S.; Hu, W.; Wei, F.; Liu, X.; Zhang, J. Indoor air pollution and lung function growth among children in four Chinese cities. Indoor Air. 2012, 22, 3–11. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, X.; Chen, X.; Cheng, Y.; Baris, E.; Ezzati, M.; China Rural, E.; Health Research, G. Exposure to indoor air pollution from household energy use in rural China: The interactions of technology, behavior, and knowledge in health risk management. Soc. Sci. Med. 2006, 62, 3161–3176. [Google Scholar] [CrossRef]
- Dicker, D.; Nguyen, G.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1684–1735. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shen, H.; Smith, K.R.; Guan, D.; Chen, Y.; Shen, G.; Liu, J.; Cheng, H.; Zeng, E.Y.; Shu, T. Estimating household air pollution exposures and health impacts from space heating in rural China. Environ. Int. 2018, 119, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Z.; Zhang, J.; Korn, L.R.; Wei, F.; Chapman, R.S. Exposure-response relationships between lifetime exposure to residential coal smoke and respiratory symptoms and illnesses in Chinese children. J. Exp. Sci. Environ. Epidemiol. 2004, 14, S78–S84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranger, M.; Potgieter-Vermaak, S.S.; Grieken, R.V. Characterization of indoor air quality in primary schools in Antwerp, Belgium. Indoor Air. 2008, 18, 454–463. [Google Scholar] [CrossRef]
- Ozgen, S.; Becagli, S.; Bernardoni, V.; Caserini, S.; Caruso, D.; Corbella, L.; Dell’Acqua, M.; Fermo, P.; Gonzalez, R.; Lonati, G.; et al. Analysis of the chemical composition of ultrafine particles from two domestic solid biomass fired room heaters under simulated real-world use. Atmos. Environ. 2017, 150, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Ielpo, P.; Fermo, P.; Comite, V.; Mastroianni, D.; Viviano, G.; Salerno, F.; Tartari, G. Chemical characterization of biomass fuel particulate deposits and ashes in households of Mt. Everest region (NEPAL). Sci. Total Environ. 2016, 573, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.; Fermo, P.; Urso, P.; Perrone, M.G.; Piazzalunga, A.; Tarlassi, J.; Carrer, P.; Cavallo, D.M.J.E.P. Particulate-bound polycyclic aromatic hydrocarbon sources and determinants in residential homes. Environ. Pollut. 2016, 218, 16–25. [Google Scholar] [CrossRef]
- Bidleman, T.F.; Billings, W.N.; Foreman, W.T. Vapor-particle partitioning of semivolatile organic compounds: Estimates from field collections. Environ. Sci. Technol. 1986, 20, 1038–1043. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, L.; Chen, S.; Amagai, T. Pollution Level, Phase Distribution and Source Analysis of Polycyclic Aromatic Hydrocarbons In Residential Air In Hangzhou, China. J. Hazard. Mater. 2009, 162, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Perspectives, T.F.J.E.H. Lung Cancer Risk after Exposure to Polycyclic Aromatic Hydrocarbons: A Review and Meta-Analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, P.; Timonen, K.L.; Tiittanen, P.; Mirme, A.; Pekkanen, R.J. Number concentration and size of particles in urban air: Effects on spirometric lung function in adult asthmatic subjects. Environ. Health Perspect. 2001, 109, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Pastorková, A.; Rössner, P. Mutagenicity monitoring of airborne particulate matter (PM10) in the Czech Republic. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 1999, 444, 373–386. [Google Scholar]
- Viras, L.G.; Athanasiou, K.; Siskos, P.A. Determination of mutagenic activity of airborne particulates and of benzo[a]pyrene concentrations in Athens atmosphere. Atmos. Environ. Part B Urban Atmos. 1990, 24, 267–274. [Google Scholar] [CrossRef]
- Xia, Z.; Duan, X.; Tao, S.; Qiu, W.; Liu, D.; Wang, Y.; Wei, S.; Wang, B.; Jiang, Q.; Song, Y.; et al. Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ. Pollut. 2013, 173, 150–156. [Google Scholar] [CrossRef]
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. Rtp. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Exposure Factors Handbook; National Service Center for Environmental Publications: Washington, DC, USA, 1996.
- Han, J.; Zhang, N.; Niu, C.; Han, B.; Bai, Z. Personal Exposure of Children to Particle-Associated Polycyclic Aromatic Hydrocarbons in Tianjin, China. Polycycl. Aromat. Compd. 2014, 34, 4. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Wang, J.; Ho, S.S.H.; He, K.; Shen, Z.; Ning, Z.; Sun, J.; Li, L.; Lei, R. Personal exposure to PM2.5-bound organic species from domestic solid fuel combustion in rural Guanzhong Basin, China: Characteristics and health implication. Chemosphere 2019, 227, 53–62. [Google Scholar] [CrossRef]
- Yury, B.; Zhang, Z.; Ding, Y.; Zheng, Z.; Wu, B.; Gao, P.; Jia, J.; Lin, N.; Feng, Y. Distribution, inhalation and health risk of PM2.5 related PAHs in indoor environments. Ecotoxicol. Environ. Saf. 2018, 164, 409–415. [Google Scholar] [CrossRef]
- Huo, C.; Sun, Y.; Liu, L.; Sverko, E.; Li, Y.; Li, W.; Ma, W.; Zhang, Z.; Song, W. Assessment of human indoor exposure to PAHs during the heating and non-heating season: Role of window films as passive air samplers. Sci. Total Environ. 2019, 659, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Noda, T.; Amagai, T.; Fusaya, M. Prediction of personal exposure to PM2.5 and carcinogenic polycyclic aromatic hydrocarbons by their concentrations in residential microenvironments. Environ. Sci. Technol. 2005, 39, 5592–5599. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Cao, J.; Li, L.; Xu, H.; Wang, T.; Wu, R.; Chen, J.; Zhang, Y.; Liu, S.; Huang, W. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Beijing: Seasonal variations, Sources, and risk assessment. J. Environ. Sci. 2019, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Bi, X.; Tan, J.; Sheng, G.; Fu, J. Seasonal variation on size distribution and concentration of PAHs in Guangzhou city, China. Chemosphere 2007, 67, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Krugly, E.; Martuzevicius, D.; Sidaraviciute, R.; Ciuzas, D.; Prasauskas, T.; Kauneliene, V.; Stasiulaitiene, I.; Kliucininkas, L. Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmos. Environ. 2014, 82, 298–306. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Liu, Y.N.; Tao, S.; Dou, H.; Zhang, T.W.; Zhang, X.L.; Dawson, R. Exposure of traffic police to Polycyclic aromatic hydrocarbons in Beijing, China. Chemosphere 2007, 66, 1920–1928. [Google Scholar] [CrossRef]
- Liu, S.; Tao, S.; Liu, W.; Liu, Y.; Dou, H.; Zhao, J.; Wang, L.; Wang, J.; Tian, Z.; Gao, Y.; et al. Atmospheric Polycyclic Aromatic Hydrocarbons in North China: A Winter-Time Study. Environ. Sci. Technol. 2007, 41, 8256–8261. [Google Scholar] [CrossRef]
- Kaur, S.; Senthilkumar, K.; Verma, V.K.; Kumar, B.; Kumar, S.; Katnoria, K.J.; Sharma, C.S. Preliminary Analysis of Polycyclic Aromatic Hydrocarbons in Air Particles (PM10) in Amritsar, India: Sources, Apportionment, and Possible Risk Implications to Humans. Arch. Environ. Contam. Toxicol. 2013, 65, 382–395. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Wu, M. Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in agricultural soil of Shanghai, China. Environ. Monit. Assess. 2011, 183, 139–150. [Google Scholar] [CrossRef]
- Chai, C.; Cheng, Q.; Wu, J.; Zeng, L.; Chen, Q.; Zhu, X.; Ma, D.; Ge, W. Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in the soils of vegetable greenhouses in Shandong, China. Ecotox. Environ. Saf. 2017, 142, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Zhang, Y.; Zhao, H.; Tan, F.; Wu, X.; Chen, J. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in the air of Dalian, China: Correlations with six criteria air pollutants and meteorological conditions. Chemosphere 2019, 216, 516–523. [Google Scholar] [CrossRef]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Marcé, R.M.; Borrull, F. Risk Assessment Related to Atmospheric Polycyclic Aromatic Hydrocarbons in Gas and Particle Phases near Industrial Sites. Environ. Health Perspect. 2011, 119, 1110–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Zhao, X. Exposure Factors Handbook of Chinese Population (Children 6–17 Years); China Environment Press: Beijing, China, 2016. [Google Scholar]
- Wang, Z.S.; Wu, T.; Duan, X.L.; Wang, S.; Zhang, X.F.; Yu, Y.J. Research on Inhalation Rate Exposure Factors of Chinese Residents in Environmental Health Risk Assessment. Res. Environ. Sci. 2009, 22, 1171–1175. [Google Scholar]
- Chen, S.C.; Liao, C.M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar]





| Definition | Units | Distribution Mode | Heating | Non-Heating | Reference |
|---|---|---|---|---|---|
| BaPeq | ng/m3 | Log-Normal | LN (2.7,0.4) | N (−2.4, 1) | Measured |
| IR | m3/d | Normal | N (35.1, 15.0) | N (42.1, 12.5) | Measured |
| EF | day/year | Constant | 140 | 220 | / |
| Exposure Duration | year | Constant | 10 | 10 | Measured |
| Average Time | day | Constant | 25500 | 25500 | / |
| Bodyweight (BW) | kg | Normal | N (29.9, 5.6) | N (29.9, 5.6) | Measured |
| Sex | N | Body Height (cm) | Body Weight (kg) | Inhalation Rate (L/min) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Heating | Boys | 7 | 138.8 | 8.7 | 31.7 | 5.1 | 41.2 | 19.4 |
| Girls | 9 | 133.8 | 8.7 | 28.5 | 5.8 | 30.4 | 9.1 | |
| Non-heating | Boys | 12 | 143.2 | 9.8 | 37.3 | 10.8 | 42.2 | 13.3 |
| Girls | 10 | 136.2 | 10 | 31.4 | 7.4 | 42 | 12.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Qin, N.; Liang, W.; Chen, X.; Hou, R.; Kang, Y.; Guo, Q.; Cao, S.; Duan, X. Polycycl. Aromatic Hydrocarbon Exposure of Children in Typical Household Coal Combustion Environments: Seasonal Variations, Sources, and Carcinogenic Risks. Int. J. Environ. Res. Public Health 2020, 17, 6520. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17186520
Liu Y, Qin N, Liang W, Chen X, Hou R, Kang Y, Guo Q, Cao S, Duan X. Polycycl. Aromatic Hydrocarbon Exposure of Children in Typical Household Coal Combustion Environments: Seasonal Variations, Sources, and Carcinogenic Risks. International Journal of Environmental Research and Public Health. 2020; 17(18):6520. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17186520
Chicago/Turabian StyleLiu, Yunwei, Ning Qin, Weigang Liang, Xing Chen, Rong Hou, Yijin Kang, Qian Guo, Suzhen Cao, and Xiaoli Duan. 2020. "Polycycl. Aromatic Hydrocarbon Exposure of Children in Typical Household Coal Combustion Environments: Seasonal Variations, Sources, and Carcinogenic Risks" International Journal of Environmental Research and Public Health 17, no. 18: 6520. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17186520






