Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways
Abstract
:1. Introduction
2. Digestate
3. Hydrothermal Carbonization
4. Hydrothermal Carbonization of Digestate
5. Hydrothermal Carbonization Process Water Characterization
5.1. pH and Color
5.2. Organic Compounds
5.3. Nutrients
5.3.1. Nitrogen
5.3.2. Phosphorus
- Al-associated P species (AlPO4, 40%);
- organic P (phytic acid, 20%);
- Fe/Ca-associated P species (ferrihydrite-adsorbed phosphate 13%, octacalcium phosphate, 16%);
- alumina-adsorbed phosphate (11%).
5.4. Heavy Metals
5.5. Toxic Compounds
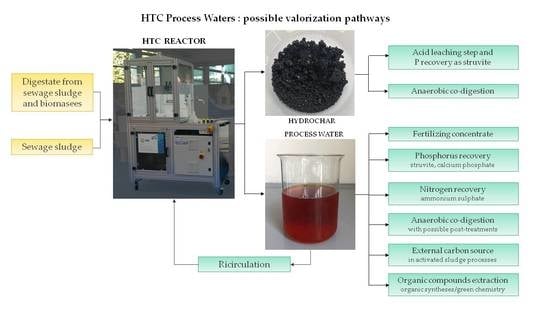
6. Hydrothermal Carbonization Process Waters Valorization
7. Industrial Implications
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NBCI National Centar for Biotechnology Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/guide/literature/ (accessed on 15 July 2020).
- Dahlin, J.; Nelles, M.; Herbes, C. Biogas digestate management: Evaluating the attitudes and perceptions of German gardeners towards digestate-based soil amendments. Resour. Conserv. Recycl. 2017, 118, 27–38. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Fuldauer, L.I.; Parker, B.M.; Yaman, R.; Borrion, A. Managing anaerobic digestate from food waste in the urban environment: Evaluating the feasibility from an interdisciplinary perspective. J. Clean. Prod. J. 2018, 185, 929–940. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Ronga, D.; Zaghi, M.; Tomasselli, A.R.; Mannella, L.; Pecchioni, N. Pelleting is a successful method to eliminate the presence of Clostridium spp. from the digestate of biogas plants. Biomass Bioenergy 2015, 81, 479–482. [Google Scholar] [CrossRef]
- Langone, M.; Ferrentino, R.; Cadonna, M.; Andreottola, G. Stoichiometric evaluation of partial nitritation, anammox and denitrification processes in a sequencing batch reactor and interpretation of online monitoring parameters. Chemosphere 2016, 164, 488–498. [Google Scholar] [CrossRef]
- Cerda, A.; Mejias, L.; Rodríguez, P.; Rodríguez, A.; Artola, A.; Font, X.; Gea, T.; Sánchez, A. Valorisation of digestate from biowaste through solid-state fermentation to obtain value added bioproducts: A first approach. Bioresour. Technol. 2019, 271, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübner, T.; Mumme, J. Integration of pyrolysis and anaerobic digestion—Use of aqueous liquor from digestate pyrolysis for biogas production. Bioresour. Technol. 2015, 183, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon NY 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Perez, S.; Sheng, K. Upgrading fuel quality of moso bamboo via low temperature thermochemical treatments: Dry torrefaction and hydrothermal carbonization. Fuel 2017, 196, 473–480. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of fruit waste: A promising technique for generating hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Sabio, E.; Alvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Davis, H.T.; Jader, L.R.; Lefebvre, P.A.; Sadowsky, M.J.; Schendel, F.J.; von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of microalgae. Biomass Bioenergy 2010, 34, 875–882. [Google Scholar] [CrossRef]
- Bach, Q.; Chen, W.; Lin, S.; Sheen, H.; Chang, J. Wet torrefaction of microalga Chlorella vulgaris ESP-31 with microwave- assisted heating. Energy Convers. Manag. 2017, 141, 163–170. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Jader, L.R.; Sadowsky, M.J.; Schendel, F.J.; von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of distiller’s grains. Biomass Bioenergy 2011, 35, 2526–2533. [Google Scholar] [CrossRef]
- Oliveira, I.; Blöhse, D.; Ramke, H.G. Hydrothermal carbonization of agricultural residues. Bioresour. Technol. 2013, 142, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.M.; Molde, J.S.; Timler, J.G.; Wood, B.M.; Mikula, A.L.; Vozhdayev, G.V.; Colosky, E.C.; Spokas, K.A.; Valentas, K.J. Phosphorus Reclamation through Hydrothermal Carbonization of Animal Manures. Environ. Sci. Technol. 2014, 48, 10323–10329. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, X.; Zhu, Y.; Peng, C.; Wang, T.; Zhu, L.; Li, C.; Zeng, G. Hydrothermal carbonization of sewage sludge: The effect of feed-water pH on fate and risk of heavy metals in hydrochars. Bioresour. Technol. 2016, 218, 183–188. [Google Scholar] [CrossRef]
- Wilk, M. A novel method of sewage sludge pre-treatment—HTC. E3S Web Conf. 2016, 10, 00103. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Funke, A.; Mumme, J.; Koon, M.; Diakité, M. Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 2013, 58, 229–237. [Google Scholar] [CrossRef]
- Aragón-briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane production from process water of sewage sludge hydrothermal carbonization. A review. Valorising sludge through hydrothermal carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 947–988. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lin, Q.M.; Zhao, X.R. The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J. Integr. Agric. 2014, 13, 471–482. [Google Scholar] [CrossRef]
- Prawisudha, P.; Namioka, T.; Yoshikawa, K. Coal alternative fuel production from municipal solid wastes employing hydrothermal treatment. Appl. Energy 2012, 90, 298–304. [Google Scholar] [CrossRef]
- Owsianiak, M.; Ryberg, M.W.; Renz, M.; Hitzl, M.; Hauschild, M.Z. Environmental Performance of Hydrothermal Carbonization of Four Wet Biomass Waste Streams at Industry-Relevant Scales. Sustain. Chem. Eng. 2016, 4, 6783–6791. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, M.R.; Iannuzzi, G.; Fabbri, C.; Senesi, N. Qualitative characterization and differentiation of digestates from different biowastes using FTIR and fluorescence spectroscopies. J. Environ. Prot. 2011, 2, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Bouallagui, H.; Marouani, L.; Hamdi, M. Performances comparison between laboratory and full-scale anaerobic digesters treating a mixture of primary and waste activated sludge. Resour. Conserv. Recycl. 2010, 55, 29–33. [Google Scholar] [CrossRef]
- Otero, M.; Lobato, A.; Cuetos, M.J.; Sánchez, M.E.; Gómez, X. Digestion of cattle manure: Thermogravimetric kinetic analysis for the evaluation of organic matter conversion. Bioresour. Technol. 2011, 102, 3404–3410. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Read, J.S.; Mattiasson, B.; Bjornsson, L. Energy production from agricultural residues: High methane yields in pilot-scale two-stage anaerobic digestion. Biomass Bioenergy 2008, 32, 44–50. [Google Scholar] [CrossRef]
- Macias-Corral, M.; Samani, Z.; Hanson, A.; Smith, G.; Funk, P.; Yu, H.; Longworth, J. Anaerobic digestion of municipal solid waste and agricultural waste and the effect of co-digestion with dairy cow manure. Bioresour. Technol. 2008, 99, 8288–8293. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Luste, S.; Heinonen-Tanski, H.; Luostarinen, S. Co-digestion of dairy cattle slurry and industrial meat-processing by-products—Effect of ultrasound and hygienization pre-treatments. Bioresour. Technol. 2012, 104, 195–201. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; He, Y.; Zhang, C.; Liu, X.; Chen, C.; Liu, G. Anaerobic co-digestion of chicken manure and corn stover in batch and continuously stirred tank reactor (CSTR). Bioresour. Technol. 2014, 156, 342–347. [Google Scholar] [CrossRef]
- Korving, L.; Van Loosdrecht, M.; Wilfert, P. Effect of Iron on Phosphate Recovery from Sewage Sludge ntroduction: The Role of Iron in Sewage Treatment. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 303–326. [Google Scholar]
- Garuti, M.; Langone, M.; Fabbri, C.; Piccinini, S. Methodological approach for trace elements supplementation in anaerobic digestion: Experience from full-scale agricultural biogas plants. J. Environ. Manag. 2018, 223, 348–357. [Google Scholar] [CrossRef]
- Möller, K.; Muller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Uysal, A.; Yilmazel, Y.D.; Demirer, G.N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef]
- Peng, W.; Pivato, A. Sustainable Management of Digestate from the Organic Fraction of Municipal Solid Waste and Food Waste Under the Concepts of Back to Earth Alternatives and Circular Economy. Waste Biomass Valorization 2019, 10, 465–481. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. Technol. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Giannis, A.; Yang, Y.; Wang, J.-Y. Products evolution during hydrothermal conversion of dewatered sewage sludge in sub- and near-critical water: Effects of reaction conditions and calcium oxide additive. Int. J. Hydrog. Energy 2015, 40, 5776–5787. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 89–124. [Google Scholar] [CrossRef] [Green Version]
- Tag, A.T.; Duman, G.; Yanik, J. Influences of feedstock type and process variables on hydrochar properties. Bioresour. Technol. 2018, 250, 337–344. [Google Scholar]
- Neethu, T.; Dubey, P. Hydrothermal carbonisation of biomass and its potential applications in various fields. Pharma Innov. J. 2018, 7, 1132–1136. [Google Scholar]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Safari, F.; Javani, N.; Yumurtaci, Z. Hydrogen production via supercritical water gasification of almond shell over algal and agricultural hydrochars as catalysts. Int. J. Hydrogen Energy 2017, 43, 1071–1080. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar]
- Karagoz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Comparative studies of oil compositions produced from sawdust, rice husk, lignin and cellulose by hydrothermal treatment. Fuel 2005, 84, 875–884. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, E. Treatment of urban sludge by hydrothermal carbonization. Bioresour. Technol. 2017, 238, 182–187. [Google Scholar] [CrossRef]
- Wirth, B.; Reza, M.T. Continuous Anaerobic Degradation of Liquid Condensate from Steam-Derived Hydrothermal Carbonization of Sewage Sludge. ACS Sustain. Chem. Eng. 2016, 4, 1673–1678. [Google Scholar] [CrossRef]
- Reza, M.T.; Becker, W.; Sachsenheimer, K.; Mumme, J. Hydrothermal carbonization (HTC): Near infrared spectroscopy and partial least-squares regression for determination of selectivecomponents in HTC solid and liquid products derived from maize silage. Bioresour. Technol. 2014, 161, 91–101. [Google Scholar] [CrossRef]
- Lu, X.J.R.; Flora, V.; Berge, N.D. Influence of process water quality on hydrothermal carbonization of cellulose. Bioresour. Technol. 2014, 154, 229–239. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lynam, J.G.; Reza, M.T.; Vasquez, V.R.; Coronella, C.J. Effect of salt addition on hydrothermal carbonization of lignocellulosic biomass. Fuel 2012, 99, 271–273. [Google Scholar] [CrossRef]
- Hu, B.; Yu, S.-H.; Wang, K.; Liu, L.; Xu, X.-W. Functional carbonaceous materials from hydrothermal carbonization of biomass: An effective chemical process. Dalt. Trans. 2008, 40, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater pH affects phosphorus transformation during hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef]
- Mumme, J.; Titirici, M.; Pfeiffer, A.; Luder, U.; Reza, M.T.; Masek, O. Hydrothermal Carbonization of Digestate in the Presence of Zeolite: Process Efficiency and Composite Properties. ACS Sustain. Chem. Eng. 2015, 3, 2967–2974. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Paulke, E.; Mumme, J.; Nehls, I. Hydrothermal carbonization of biomass: Major organic components of the aqueous phase. Chem. Eng. Technol. 2014, 37, 511–518. [Google Scholar] [CrossRef]
- Yu, Y.; Lei, Z.; Yuan, T.; Jiang, Y.; Chen, N.; Feng, C.; Shimizu, K.; Zhang, Z. Simultaneous phosphorus and nitrogen recovery from anaerobically digested sludge using a hybrid system coupling hydrothermal pretreatment with MAP precipitation. Bioresour. Technol. 2017, 243, 634–640. [Google Scholar] [CrossRef]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: A feasibility study on a laboratory scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Yang, Y.; Amaniampong, P.N.; Wang, J.-Y. Effective Nitrogen Removal and Recovery from Dewatered Sewage Sludge Using a Novel Integrated System of Accelerated Hydrothermal Deamination and Air Stripping. ACS Sustain. Chem. Eng. 2015, 49, 6872–6880. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; He, C.; Chen, X.; Lapkin, A.A.; Xiao, W.; Wang, C.-H. Nitrogen Removal and Energy Recovery from Sewage Sludge by Combined Hydrothermal Pretreatment and CO2 Gasification. ACS Sustain. Chem. Eng. 2018, 6, 16629–16636. [Google Scholar] [CrossRef]
- Shi, W.; Feng, C.; Huang, W.; Lei, Z.; Zhang, Z. Study on interaction between phosphorus and cadmium in sewage sludge during hydrothermal treatment by adding hydroxyapatite. Bioresour. Technol. 2014, 159, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Liu, C.; Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour. Technol. 2013, 137, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Alatalo, S.M.; Repo, E.; Mäkilä, E.; Salonen, J.; Vakkilainen, E.; Sillanpää, M. Adsorption behavior of hydrothermally treated municipal sludge pulp and paper industry sludge. Bioresour. Technol. 2013, 147, 71–76. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Evolution of phosphorus complexation and mineralogy during (hydro)thermal treatments of activated and anaerobically digested sludge: Insights from sequential extraction and P K-edge XANES. Water Res. 2016, 100, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Wirth, B.; Reza, T.; Mumme, J. Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef]
- Yu, Y.; Lei, Z.; Yang, X.; Yang, X.; Huang, W.; Shimizu, K.; Zhang, Z. Hydrothermal carbonization of anaerobic granular sludge: Effect of process temperature on nutrients availability and energy gain from produced hydrochar. Appl. Energy 2018, 229, 88–95. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef]
- Wirth, B.; Mumme, J. Anaerobic Digestion of Waste Water from Hydrothermal Carbonization of Corn Silage. Appl. Bioenergy 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Nuchdang, S.; Frigon, J.; Roy, C.; Pilon, G.; Phalakornkule, C.; Guiot, S.R. Hydrothermal post-treatment of digestate to maximize the methane yield from the anaerobic digestion of microalgae. Waste Manag. 2018, 71, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Coronella, C.; Holtman, K.M.; Franqui-Villanueva, D.; Poulson, S.R. Hydrothermal Carbonization of Autoclaved Municipal Solid Waste Pulp and Anaerobically Treated Pulp Digestate. ACS Sustain. Chem. Eng. 2016, 4, 3649–3658. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 99, 360–373. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour. Technol. 2016, 200, 991–998. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Nelles, M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour. Technol. 2018, 260, 404–412. [Google Scholar] [CrossRef]
- Qiao, W.; Peng, C.; Wang, W.; Zhang, Z. Biogas production from supernatant of hydrothermally treated municipal sludge by upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2011, 102, 9904–9911. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Speciation Dynamics of Phosphorus during (Hydro)Thermal Treatments of Sewage Sludge. Environ. Sci. Technol. 2015, 49, 14466–14474. [Google Scholar] [CrossRef]
- Liang, Y.J.; Chai, L.Y.; Liu, H.; Min, X.B.; Mahmood, Q.; Zhang, H.J.; Ke, Y. Hydrothermal sulfidation of zinc-containing neutralization sludge for zinc recovery and stabilization. Miner. Eng. 2012, 25, 14–19. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.; Lu, X.; Liu, C. Mineralization Behavior of Fluorine in Perfluorooctanesulfonate (PFOS) during Thermal Treatment of Lime-Conditioned Sludge. Environ. Sci. Technol. 2013, 47, 2621–2627. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, W.; Cheng, L.; Sun, B.; Sun, D.; Bi, J. Hydrogen production from lignite via supercritical water in flow-type reactor. Int. J. Hydrogen Energy 2010, 35, 11810–11815. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Schmahl, W.W.; Müllejans, T. Hydrothermal processing of MSWI Fly Ash-towards new stable minerals and fixation of heavy metals. J. Hazard. Mater. 2009, 167, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Poerschmann, J.; Weiner, B.; Wedwitschka, H.; Baskyr, I.; Koehler, R.; Kopinke, F.D. Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresour. Technol. 2014, 164, 162–169. [Google Scholar] [CrossRef]
- vom Eyser, C.; Palmu, K.; Schmidt, T.C.; Tuerk, J. Pharmaceutical load in sewage sludge and biochar produced by hydrothermal carbonization. Sci. Total Environ. 2015, 537, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Weiner, B.; Poerschmann, J.; Wedwitschka, H.; Koehler, R.; Kopinke, F.-D. Influence of Process Water Reuse on the Hydrothermal Carbonization of Paper. ACS Sustain. Chem. Eng. 2014, 2, 2165–2171. [Google Scholar] [CrossRef]
- Catalkopru, A.K.; Kantarli, I.C.; Yanik, J. Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 2017, 226, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lynam, J.G.; Reza, M.T.; Yan, W.; Vásquez, V.R.; Coronella, C.J. Hydrothermal carbonization of various lignocellulosic biomass. Biomass Convers. Biorefinery 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Li, F.; Liu, L.; An, Y.; He, W.; Themelis, N.J.; Li, G. Hydrothermal liquefaction of three kinds of starches into reducing sugars. J. Clean. Prod. 2016, 112, 1049–1054. [Google Scholar] [CrossRef]
- Stemann, J.; Erlach, B.; Ziegler, F. Hydrothermal Carbonisation of Empty Palm Oil Fruit Bunches: Laboratory Trials, Plant Simulation, Carbon Avoidance, and Economic Feasibility. Waste Biomass Valorization 2013, 4, 441–454. [Google Scholar] [CrossRef]
- Uddin, M.H.; Reza, M.T.; Lynam, J.G.; Coronella, C.J. Effects of Water Recycling in Hydrothermal Carbonization of Loblolly Pine. Environ. Prog. Sustain. Energy 2014, 33, 1309–1315. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product ? Waste Biomass Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. Production of bio-coal, bio-methane and fertilizer from seaweed via hydrothermal carbonisation. Algal Res. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Hognon, C.; Delrue, F.; Texier, J.; Grateau, M.; Thiery, S.; Miller, H.; Roubaud, A. Comparison of pyrolysis and hydrothermal liquefaction of Chlamydomonas reinhardtii. Growth studies on the recovered hydrothermal aqueous phase. Biomass Bioenergy 2015, 73, 23–31. [Google Scholar] [CrossRef]
- Yao, C.; Pan, Y.; Lu, H.; Wu, P.; Meng, Y.; Cao, X.; Xue, S. Utilization of recovered nitrogen from hydrothermal carbonization process by Arthrospira platensis. Bioresour. Technol. 2016, 212, 26–34. [Google Scholar] [CrossRef]
- Vozhdayev, G.V.; Spokas, K.A.; Molde, J.S.; Heilmann, S.M.; Wood, B.M.; Valentas, K.J. Response of maize germination and growth to hydrothermal carbonization filtrate type and amount. Plant Soil 2015, 396, 127–136. [Google Scholar] [CrossRef]
- Fregolente, L.G.; Miguel, T.B.A.R.; de Castro Miguel, E.; de Almeida Melo, C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Toxicity evaluation of process water from hydrothermal carbonization of sugarcane industry by-products. Environ. Sci. Pollut. Res. 2019, 26, 27579–27589. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Shi, Z.-J.; Xu, F.; Sun, R. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Zhou, Y.; Guo, W.; Jiang, H.; Xu, Q. Characterization of hydrothermal carbonization products (hydrochars and spent liquor) and their biomethane production performance. Bioresour. Technol. 2018, 267, 9–16. [Google Scholar] [CrossRef]
- Wang, D.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef]
- Wood, B.M.; Jader, L.R.; Schendel, F.J.; Hahn, N.J.; Valentas, K.J.; McNamara, P.J.; Novak, P.M.; Heilmann, S.M. Industrial symbiosis: Corn ethanol fermentation, hydrothermal carbonization, and anaerobic digestion. Biotechnol. Bioeng. 2013, 110, 2624–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyktari, E.; Danso-Boateng, E.; Wheatley, A.; Holdich, R. Anaerobic digestion of liquid products following hydrothermal carbonisation of faecal sludge at different reaction conditions. Desalin. Water Treat. 2017, 91, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Wirth, B.; Krebs, M.; Andert, J. Anaerobic degradation of increased phenol concentrations in batch assays. Environ. Sci. Pollut. Res. 2015, 22, 19048–19059. [Google Scholar] [CrossRef] [PubMed]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Weide, T.; Brügging, E.; Wetter, C. Anaerobic and aerobic degradation of wastewater from hydrothermal carbonization in a continuous, three-stage, semi-industrial system. J. Environ. Chem. Eng. 2019, 7, 102912. [Google Scholar] [CrossRef]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel approach of phosphate-reclamation as struvite from sewage sludge by utilising hydrothermal carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]



| Reference | Alburquerque et al. (2012) [53] | Alburquerque et al. (2012) [53] | Alburquerque et al. (2012) [53] | Alburquerque et al. (2012) [53] | Uysal et al. (2010) [54] | a Peng and Pivato, (2019) [55] b Tampio et al. (2016) [56] |
|---|---|---|---|---|---|---|
| Digestate | Pig Slurry and Energy-Crop Residues | Pig Slurry and Animal By-Products | Cattle Manure and Glycerin | Cattle Manure and Agro-Industrial Residues | Municipal Sewage Sludge | Organic Solid Waste |
| pH | 7.80–7.90 | 7.86–8.20 | 5.64–7.35 | 7.50–7.90 | 7.6 | 7.60–8.30 a |
| EC [dS m−1] | 23.3–26.0 | 21.1–30.8 | 5.20–14.5 | 8.7–25.7 | - | - |
| TS [g L−1] | 28.3–43.9 | 19.5–29.5 | 17.6–72.9 | 17.6–90.1 | 25.3 ± 0.2 | 7.2–78.8 a |
| TOC [g L−1] | 8.3–14.7 a | 5.8–8.4 | 8.3–42.8 | 5.8–33.7 | - | - |
| COD [g L−1] | 3.7–4.3 | 1.2–3.5 | 8.2–27.6 | 1.0–5.4 | 25.8 ± 1.9 | 21.8–100.3 b |
| BOD5 [g L−1] | 4.0–6.5 | 2.2–6.2 | 10.6–52.5 | 1.2–5.9 | 0.4 ± 0.03 (as SCOD) | 7.3–15.4 (as SCOD) b |
| TN [g L−1] | 3.4–3.6 | 2.9–4.9 | 0.6–2.3 | 1.4–4.0 | 1.0 ± 0.02 | 4.7–8.7 b |
| NH4+-N [g L−1] | 2.6–2.9 | 2.2–3.5 | 0.4 – 1.0 | 0.8–2.4 | 0.9 ± 0.01 | 1.7–27.5 a 1.7–4.5 b |
| TP [g L−1] | 1.2–1.2 | 0.2–0.8 | 0.8–1.8 | 0.2–0.8 | 0.39 ± 0.003 | - |
| PO4−-P [g L−1] | - | - | - | - | 0.021 ± 0.0 | - |
| K [g L−1] | 2.7–3.1 | 2.0–3.1 | 0.8–1.8 | 1.1–3.1 | 0.074 ± 0.005 | - |
| Al [mg L−1] | - | - | - | - | 91 ± 10 | - |
| S [mg L−1] | 367–417 | 219–680 | 48–265 | 113–457 | - | - |
| Ca [mg L−1] | 1863–1993 | 218–828 | 192–1753 | 1008–4026 | 1049 ± 57 | - |
| Mg [mg L−1] | 633–721 | 67–365 | 79–333 | 257–698 | 194 ± 1.5 | - |
| Na [mg L−1] | 666–699 | 696–995 | 66–1842 | 276–746 | 175 ± 8.2 | - |
| Cl [mg L−1] | 1495–1613 | 1598–2120 | 448–685 | 452–1418 | - | - |
| Fe [mg L−1] | 143–224 | 22–63 | 95–165 | 30–301 | 318 ± 32.5 | - |
| Mn [mg L−1] | 23–31 | 2.9–15.4 | 3.2–17.1 | 6.0–27.5 | 3.6 ± 0.1 | - |
| Zn [mg L−1] | 45.9–62.5 | 34.7–140.2 | 10.6–28.3 | 7.7–27.7 | 51 ± 5.4 | 56–300 a |
| Cu [mg L−1] | 7.0–8.4 | 4.0–15.1 | 1.4–13.0 | 2.8–10.8 | 4.0 ± 0.1 | 14–80 a |
| B [mg L−1] | 2.7–3.2 | 2.2–3.1 | 1.3–4.8 | 1.7–3.5 | - | - |
| HTC Feedstock | Laboratory Treatment Prior to HTC | Reactor Volume | HTC Conditions | Studied Products/Characteristics | Reference |
|---|---|---|---|---|---|
| ADSS | - | 160 mL | 250 °C, 20 h | Process waters Hydrochar | Berge et al. (2011) [23] |
| ADSS | - | 500 mL | 160, 220, 250 °C, 30 min | Process waters Hydrochar | Aragón-briceño et al. (2017) [34] |
| ADSS | - | 200 mL | 120–240 °C, 1–60 min | Process waters Hydrochar MAP precipitation | Yu et al. (2017) [81] |
| Dewatered ADSS (solid) | Water dilution Use of CaO additive | 1000 mL | 200 to 380 °C, 20 min 500 rpm | Process waters Hydrochar MAP precipitation | He et al. (2015) [59] |
| Dewatered ADSS (solid) | Water dilution in order to obtain a TS content of 20% Use of citric acid as catalyst | 25.0 L | 205 °C, 7 h pH regulation by acetic acid and sodium hydroxide | Process waters Hydrochar Dewaterability | Escala et al. (2013) [82] |
| Dewatered ADSS (solid) | Pre-dried at 105 °C for 12 h Water dilution | 1000 mL | 200 to 380 °C, 20 min 500 rpm | Process waters Hydrochar Ammonia stripping | He et al. (2015) [83] |
| Dewatered ADSS (solid) | - | 200 mL | 160, 200, 240 °C, 4, 8, 12 h | Process waters Hydrochar N distribution | Shen et al. (2018) [84] |
| Dewatered ADSS (solid) | Water dilution Addition of Cd | 200 mL | 200, 280 °C, 1 h | Process waters Hydrochar P distibution | Shi et al. (2014) [85] |
| Dewatered ADSS (solid) | Water dilution Addition of Cr, Ni, Cu, Zn, Cd, Pb | 200 mL | 170, 200. 280 °C, 1 h | Process waters Hydrochar P and HM distribution | Shi et al. (2013) [86] |
| Dewatered ADSS (solid) | - | 125 mL | 200 °C, 4, 6, 8, 10, 12 h | Hydrochar | He et al. (2013) [28] |
| ADSS | Water dilution | 1000 mL | 180–200 °C, 30 min 200 rpm | Hydrochar Dewaterability | Kim et al. (2014) [32] |
| ADSS | Water dilution | 200 mL | 200 °C, 24 h | Hydrochar | Alatalo et al. (2013) [87] |
| Dewatered ADSS (solid) | Pre-dried at 105 °C for 24 h Triturated | 500 mL | 200, 230, 260 °C, 2 h | Hydrochar P evolution | Wang et al. (2017) [78] |
| Dewatered ADSS (solid) | Water dilution | 20 mL | 225°C, 4–16 h | Hydrochar P distribution | Huang and Tang (2016) [88] |
| ADSS | 3.0 m3 | 200 °C, 6 h pH regulation by citric acid | Process waters HTC + AD | Wirth et al. (2015) [89] | |
| AGS | - | 200 mL | 160, 200, 240 °C, 1 h | Process waters Hydrochar AD+HTC | Yu et al. (2018) [90] |
| ADSS | 3.0 m3 | 200 °C, 6 h pH regulation by citric acid | Condensate | Wirth and Reza (2016) [70] | |
| Anaerobically digested wheat straw (thermophilic digestion) | Pre-dried at 60 °C for 72 h Water dilution in order to obtain a carbon concentration of 26.6 g L−1 | 1000 mL | 190, 210, 230, 250 °C, 1, 2.5, 4 h | Hydrochar | Funke et al. (2013) [33] |
| Anaerobically digested maize silage (thermophilic digestion) | Water dilution in order to obtain a carbon concentration of 42.3 g L−1 | 1000 mL | 190, 230, 270 °C, 2, 6, 10 h 90 rpm pH regulation by citric acid (pH 3, 5, 7) | Hydrochar | Mumme et al. (2011) [10] |
| Anaerobically digested wheat straw (thermophilic digestion) | Water dilution in order to obtain a TS content of 10% | 1000 mL | 230 °C, 6 h 90 rev/min | Hydrochar | Mumme et al. (2014) [66] |
| Dried anaerobically digested cow manure and maize (mass ratio of 4:3 as feedstock) and zeolite | Pre-dried at 105 °C for 24 h Cut to particle size below 1 mm Water dilution | 1000 mL | 190, 230, 270 °C, 2 h 90 rev/min Addition of zeolite | Hydrochar–zeolite composite | Mumme et al. (2015) [79] |
| Anaerobically digested agro-industrial biomass | Pre-dried at 60 °C for 48 h | 75 mL | 250 °C, 1 h | Process waters Hydrochar | Ekpo et al. (2016) [91] |
| Anaerobically digested wheat straw (thermophilic digestion) | Pre-dried at 60 °C for 48 h Water dilution in order to obtain a carbon concentration of 26.7 g L−1 | 125 mL | 190, 230, 250, 270 °C, 6.0 h | Process waters | Becker et al. (2014) [80] |
| Anaerobically digested corn silage | - | Full-scale plant located at Karlsruhe, Germany | 220 °C, 6.0 h | Process waters Valorization process waters througth AD | Wirth and Mumme (2014) [92] |
| Dewatered anaerobically digested algal biomass | Water dilution | 300 mL | 200 °C, 1 h | Process waters HTC + AD system | Nuchdang et al. (2018) [93] |
| Dewatered anaerobically digested agro-industrial biomass | 25.0 L | 180 °C, 4 h | Process waters Hydrochar | Oliveira et al. (2013) [26] | |
| Dewatered anaerobically digested municipal solid waste | - | 100 mL | 200, 250, 300 °C 0.5, 2 h 90 rpm | Process waters, Hydrochar | Reza et al. (2016) [94] |
| Anaerobically digested corn silage | Full-scale plant, Germany | 180 °C, 8–10 h | Process water Hydrochar Toxicity | Bargmann et al. (2013) [95] |
| References | Raw Material and HTC Conditions | Yield | pH | TOC | Soluble COD | VFAs | Acetic Acid | TS | Total N | NH4+-N | Total Soluble P | Ortho-P | Total K | Phenols | Others | C | H | N | S | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | mgC L−1 | mg L−1 | mgCOD L−1 | mg L−1 | % | mgN L−1 | mgN L−1 | mgP L−1 | mgP L−1 | mgK L−1 | % | % | % | % | % | |||||
| Berge et al. (2011) [23] | ADSS | |||||||||||||||||||
| HTC 250 °C, 20 h | - | 8.0 | 4000 | 10000 | YES 1 | YES 1 | ||||||||||||||
| Aragón-briceño et al. (2017) [34] | ADSS | 7.8 | 461.6 | 1843 | 4.8 | 4.5 | 1493 | 1344 | 91.3 | 80.1 | 30.5 | 4.4 | 10.2 | 0.7 | 54.1 | |||||
| HTC 160 °C, 30 min | 9.1 | 4686 | 12,642 | 191.1 | 2066 | 1258 | 94.0 | 53.9 | 45.8 | 6.8 | 11.1 | 1.9 | 34.5 | |||||||
| HTC 220 °C, 30 min | 7.1 | 4584 | 12,992 | 406.0 | 2191 | 1704 | 72.6 | 59.8 | 49.2 | 6.3 | 12.3 | 2.4 | 29.8 | |||||||
| HTC 250 °C, 30 min | 8.1 | 4879 | 12,164 | 715.7 | 2354 | 1685 | 103.8 | 56.8 | 68.0 | 6.6 | 6.6 | 1.8 | 10.9 | |||||||
| He et al. (2015) [59] | Dewatered ADSS | 17.5 | ||||||||||||||||||
| HTC 200 °C, 20 min | 87 (%vol) | 8.6 | 24,070 | 63,900 | 12,000 | 4020 | 246 | YES 1 | ||||||||||||
| HTC 280 °C, 20 min | 98 (%vol) | 8.4 | 16,000 | 40,000 | 10,100 | 6400 | 191 | |||||||||||||
| HTC 380 °C, 20 min | 95(%vol) | 8.1 | 12,510 | 30,400 | 10,000 | 7980 | 89 | |||||||||||||
| HTC 380 °C, 20min, CaO | 10.0 | 18,630 | 55,800 | 12,000 | 8700 | 21 | ||||||||||||||
| Escala et al. (2013) [82] | Dewatered ADSS | 6.9–7.4 | 23.9 | |||||||||||||||||
| HTC 205°C, 7h, Ca | 7.0 | 53,000 | 2590 | 2047 | 14.3 | 11.5 | 666 | |||||||||||||
| HTC 205°C, 7h | 6.9 | 40,600 | 2710 | 2153 | 17.8 | 4.8 | 633 | |||||||||||||
| Shi et al. (2014) [85] | Dewatered ADSS | 6.4 | 15 | 3150 | 3900 | |||||||||||||||
| HTC 200 °C, 1h | 0.2%+ | |||||||||||||||||||
| HTC 280 °C, 1h | 0.6%+ | |||||||||||||||||||
| Yu et al. (2017) [81] | ADSS | 6.3 | 1800 | 1.8 | 300 | 370 | ||||||||||||||
| HTC 160 °C, 30 min | 6.0 | 4000 | 400 | 480 | ||||||||||||||||
| HTC 200 °C, 30 min | 5.7 | 5000 | 450 | 570 | ||||||||||||||||
| HTC 240 °C, 30 min | 5.5 | 6000 | 490 | 400 | ||||||||||||||||
| Shi et al. (2013) [86] | Dewatered ADSS | 6.4 | 14.5 | |||||||||||||||||
| HTC 170 °C, 1 h | 7.6 | 2357 | 12.5 | |||||||||||||||||
| HTC 200 °C, 1h | 8.5 | 2586 | 15.8 | |||||||||||||||||
| HTC 280 °C, 1 h | 9.2 | 3566 | 30.4 | |||||||||||||||||
| Wirth et al. (2015) [89] | ADSS | |||||||||||||||||||
| HTC 200 °C, 6 h, pH regulation | 4.7 | 13,400 | 34300 | 2060 | 3.4 | 2800 | 1000 | YES 2 | ||||||||||||
| Yu et al., (2018) [90] | AGS | 6.8 | 1118 | 100 | 0 | 9.5 | ||||||||||||||
| HTC 160 °C, 1 h | 6.0 | 15,611 | 454 | 300 | ||||||||||||||||
| HTC 200 °C, 1 h | 5.8 | 1100 | 900 | |||||||||||||||||
| HTC 240 °C, 1 h | 5.6 | 2557 | 2000 | |||||||||||||||||
| Ekpo et al. (2016) [91] | Agro-industrial digestate | |||||||||||||||||||
| HTC 250 °C, 1 h | 30 (wt%) | 7.7 | 62,350 | 18,610 | 10,235 | 840 | 2340 | |||||||||||||
| Becker et al. (2014) [80] | Wheat straw digestate | |||||||||||||||||||
| HTC 190 °C, 6.0 h | 4.0 | 5800 | 1000 | YES 3 | YES 3 | |||||||||||||||
| HTC 230 °C, 6.0 h | 4.0 | 9000 | 1000 | |||||||||||||||||
| HTC 250 °C, 6.0 h | 4.0 | 7800 | 1250 | |||||||||||||||||
| HTC 270 °C, 6.0 h | 4.0 | 9500 | 1200 | |||||||||||||||||
| Wirth and Mumme (2014) [92] | Corn silage digestate | |||||||||||||||||||
| HTC 220 °C, 6.0 h | 3.88 | 15,660 | 41,350 | 5260 | 2.8 | 685 | 229 | 197 | 290 | |||||||||||
| Nuchdang et al. (2018) [93] | Microalgae digestate | 1926 | 0.9 | |||||||||||||||||
| HTC 200 °C, 1.0 h | 8204 | 0.9 | ||||||||||||||||||
| Reza et al. (2016) [94] | MSW digestate | 8.1 | 23 | |||||||||||||||||
| HTC 200 °C, 30 min | 8.2 | |||||||||||||||||||
| HTC 200 °C, 2.0 h | 8.3 | |||||||||||||||||||
| Bargmann et al. (2013) [95] | Corn silage digestate | |||||||||||||||||||
| HTC 180 °C, 9.0 h | 5.7 | 83.3 | 20.3 | 328 |
| Compound | Application |
|---|---|
| 1-Methyl-4-[nitromethyl]-4-piperidinol | Production of antitumor agents and products involved in the treatment of cardiovascular diseases |
| 1-Methyldodecylamine | Preparation of N,N,N,N,N,N- trimethyldodecylammonium bromide |
| 1-Phenethyl-piperidin-4-ol | - |
| 1-Propanol, 2-amino- | Organic syntheses (e.g, Schiff base ligands) |
| 2,5-Pyrrolidinedione, 1-ethyl- | Organic syntheses |
| 2,5-Pyrrolidinedione, 1-methyl- | Organic syntheses, as well as in some industrial silver-plating processes |
| 2-Butanamine, (S) | Production of some pesticides |
| 2-Cyclopenten-1-one, 2,3-dimethyl- | - |
| 2-Cyclopenten-1-one, 2-methyl- | - |
| 2-Heptanamine, 5-methyl- | - |
| 2-Propanamine | Production of some herbicides and pesticides including atrazine, bentazon, glyphosate; agent for plastics; intermediate in organic synthesis of coating materials, pesticides, plastics, rubber chemicals, pharmaceuticals and others; additive in the petroleum industry |
| 3-Aminopyridine | Synthesis of organic ligand 3-pyridylnicotinamide. |
| 3-Buten-2-one, 3-methyl-, dimethylhydrazone | - |
| 3-Cyclohexene-1-carboxaldehyde, 4-methyl- | - |
| 4-Fluorohistamine | Organic syntheses |
| Acetic acid | Production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibers and fabrics; descaling agent, used in the food industry, in biochemistry |
| Benzoic acid, 2,4-dihydroxy-, (3-diethylamino-1- methyl)propyl ester | - |
| Dimethylamine | Dehairing agent in tanning, in dyes, in rubber accelerators, in soaps and cleaning compounds; agricultural fungicide |
| dl-Alanine | Food and pharmaceutical industry; plating chemicals and animal feed |
| Formic acid phenyl ester | Used for palladium-catalyzed carbonylation of aryl, alkenyl and allyl halides; used as a reagent for the formulation of amines |
| Hydrogen chloride | Used in cleaning, pickling, electroplating metals, tanning leather, and refining and as an agent for producing a wide variety of products |
| Methylpent-4-enylamine | - |
| Phenethylamine, p-methoxy-.alpha.-methyl-, (.+/-.)- | - |
| Phenol | Precursor to many materials and useful compounds; used to synthesize plastics and related materials; production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. |
| Phenol, 4-methyl- | Production of antioxidants, e.g., butylated hydroxytoluene |
| Pyrazole, 1-methyl-4-nitro- | - |
| Tetrahydro-4H-pyran-4-ol | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langone, M.; Basso, D. Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways. Int. J. Environ. Res. Public Health 2020, 17, 6618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17186618
Langone M, Basso D. Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways. International Journal of Environmental Research and Public Health. 2020; 17(18):6618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17186618
Chicago/Turabian StyleLangone, Michela, and Daniele Basso. 2020. "Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways" International Journal of Environmental Research and Public Health 17, no. 18: 6618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17186618