Improvement of Ficin-Based Inhibitive Enzyme Assay for Toxic Metals Using Response Surface Methodology and Its Application for Near Real-Time Monitoring of Mercury in Marine Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Casein and Ficin Solution
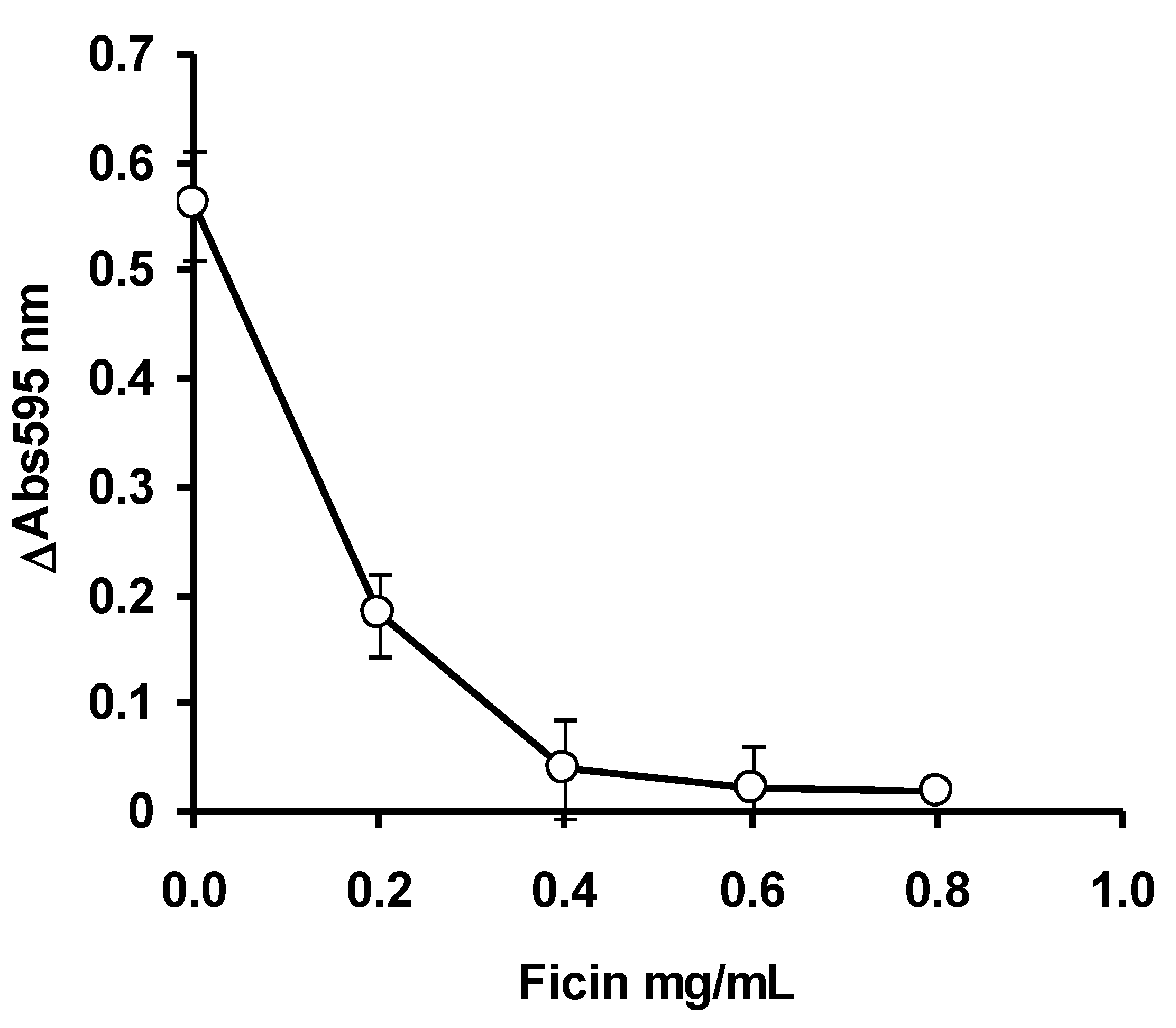
2.2. Ficin Optimization Studies
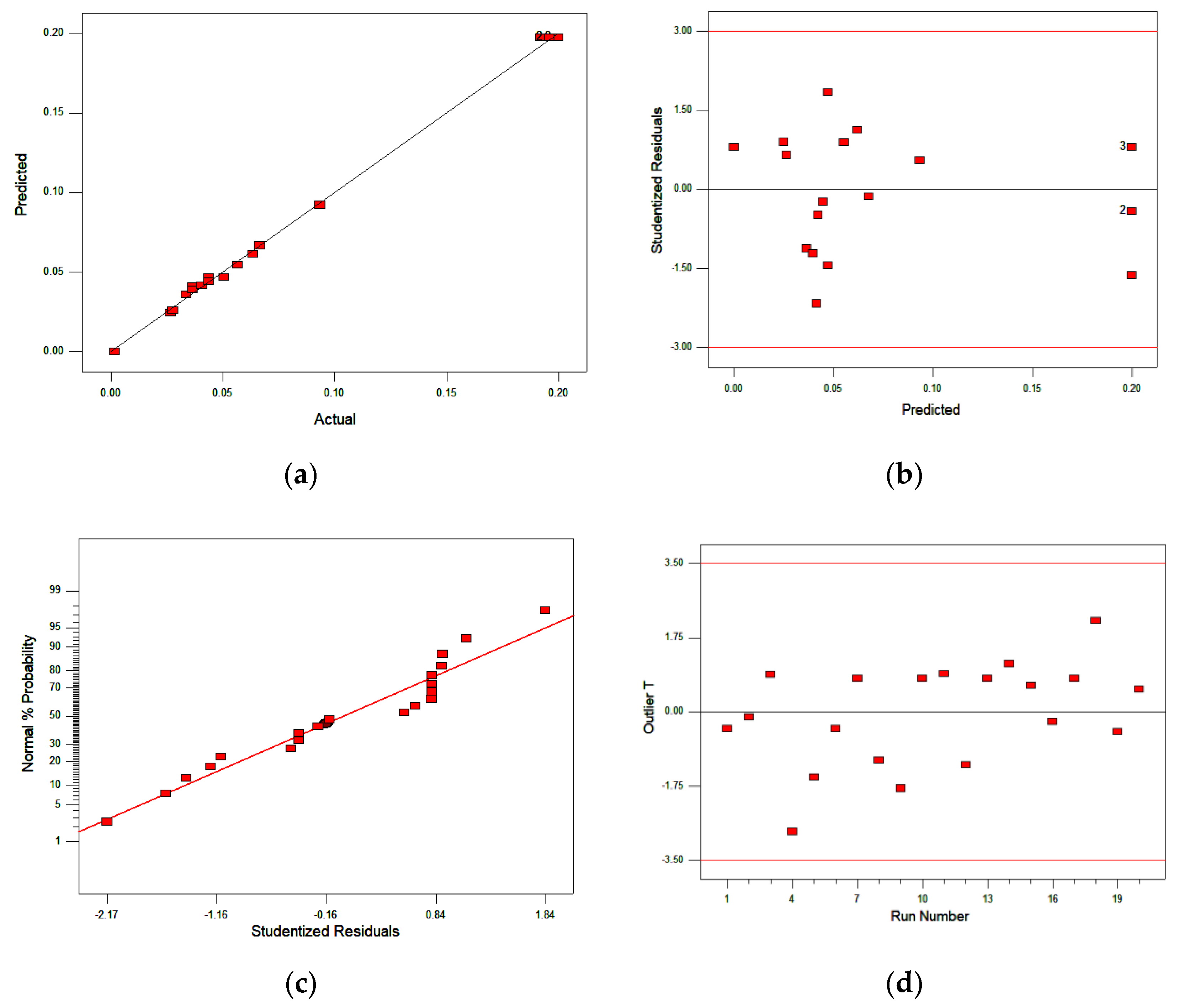
2.3. Optimization Using RSM
Central Composite Design Experiments
2.4. Ficin Mercury Inhibition Studies
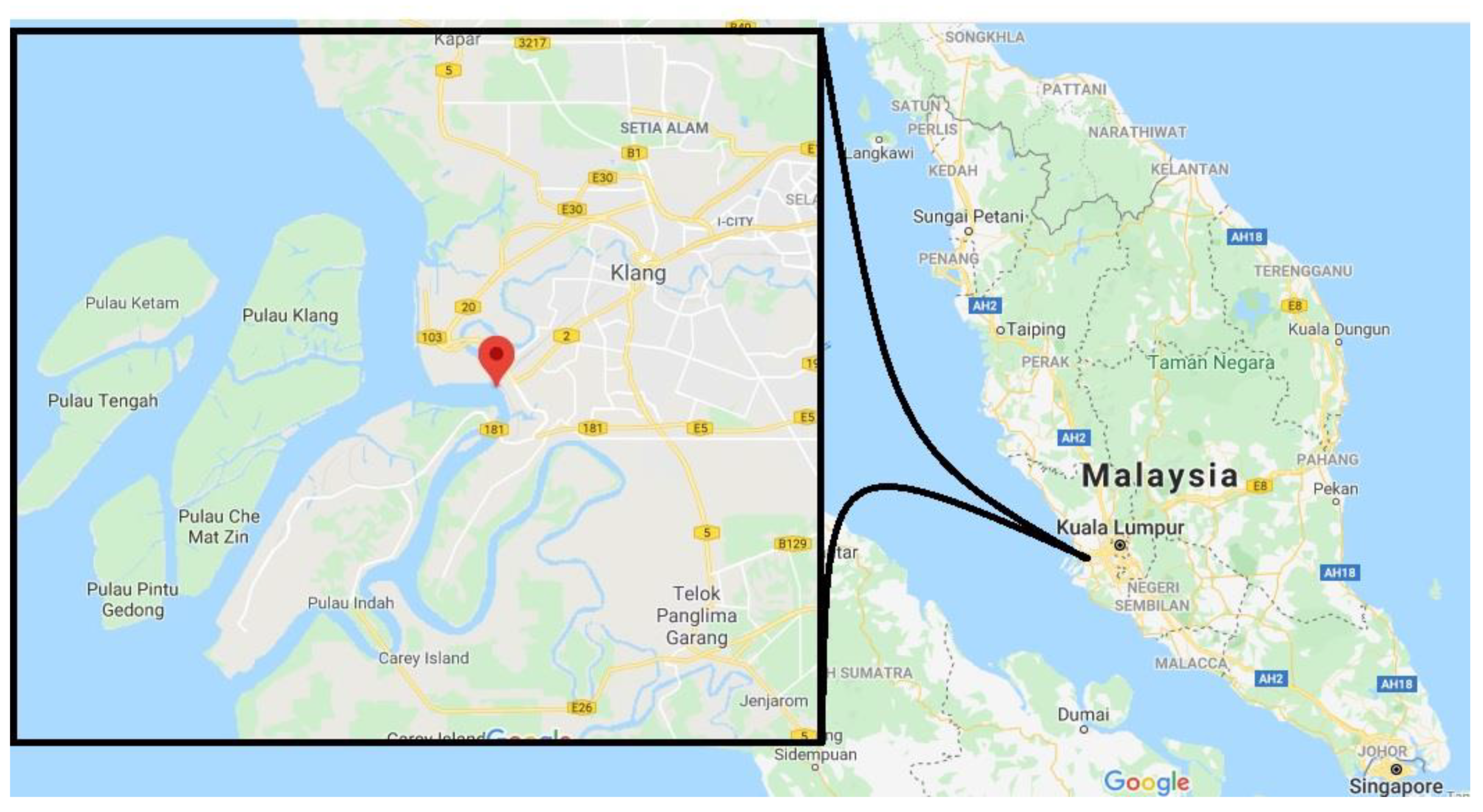
2.5. Field Trials
2.6. Data and Statistical Analysis
Test Activity of Control
3. Result and Discussion
3.1. Optimization Using OFAT
3.2. Optimization Using Response Surface Methodology (RSM)
− 0.058 * A * A − 0.057 * B * B − 0.042 * C * C + 9.750E − 003 * A * B − 4.750E − 003 * A * C + 8.250E − 003 * B * C
0.10293 * Casein − 0.058263 * pH2 − 0.28191 * Ficin * Ficin − 0.018634 * Casein * Casein +
0.021667 * pH * Ficin − 0.00032 * pH * Casein + 0.012222 * Ficin * Casein
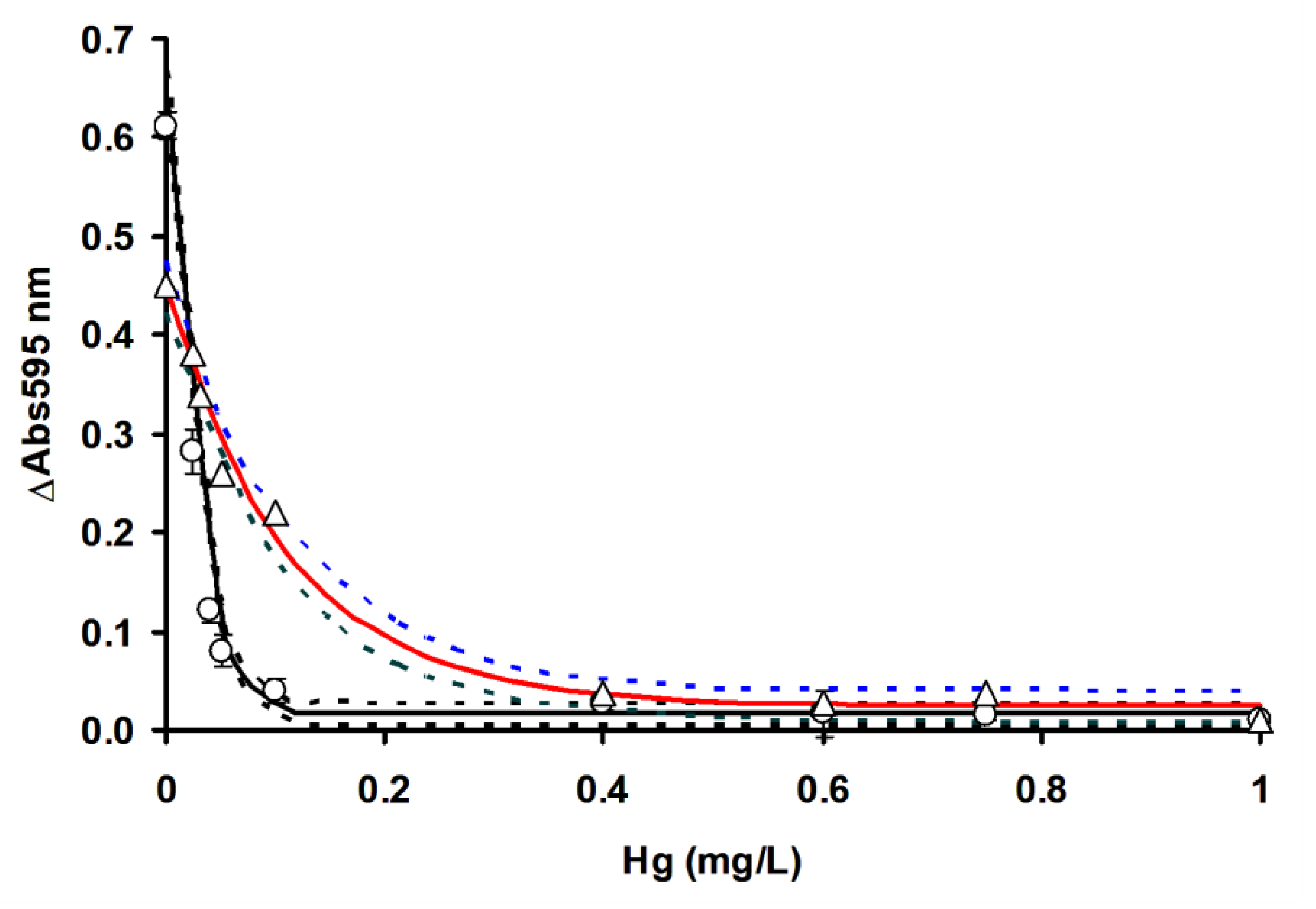
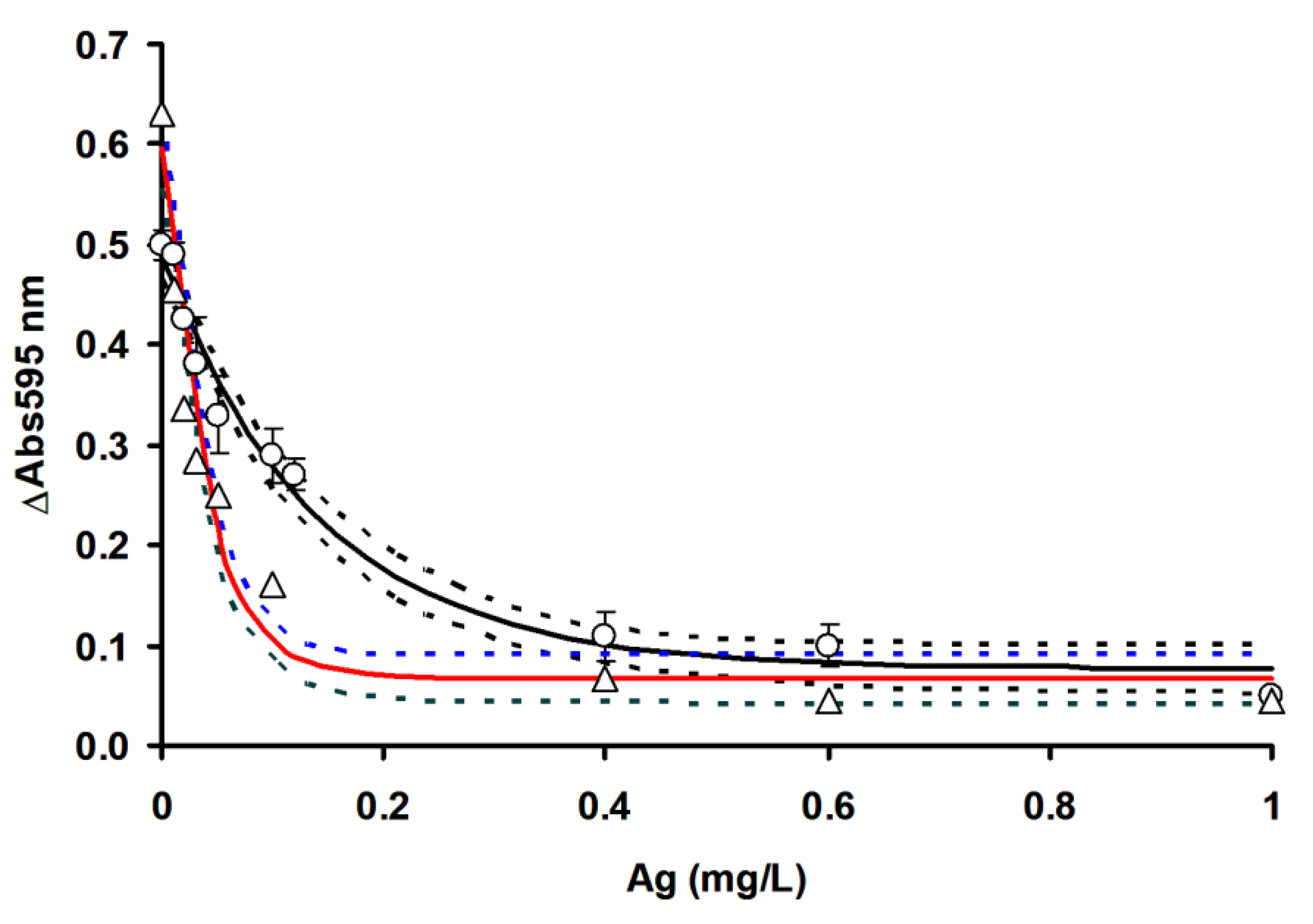
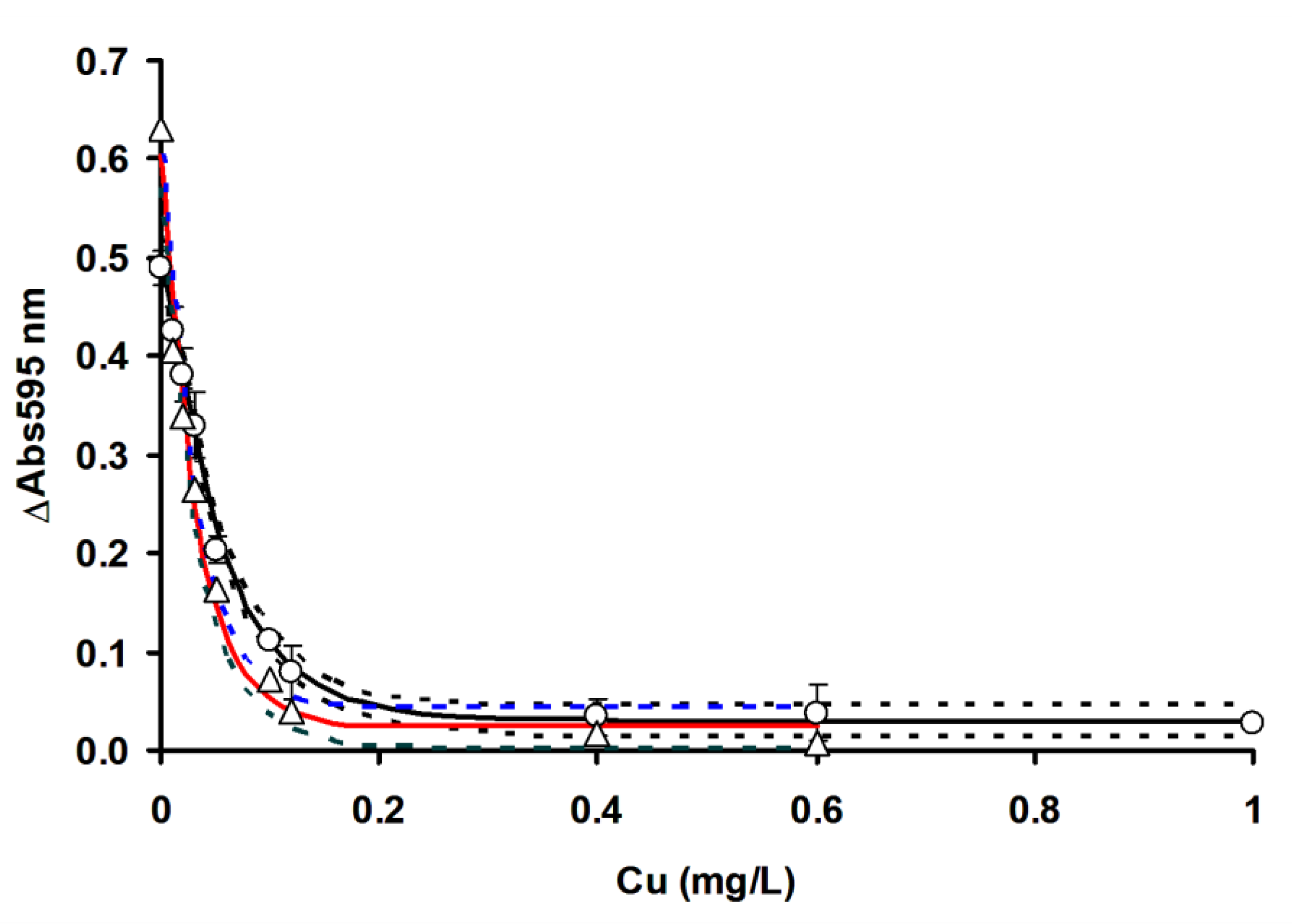
3.3. Comparison of OFAT and RSM in Mercury Detection Using Ficin
3.4. Near Real-Time Field Trials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Department of Environment. Malaysian Marine Water Quality Standards; Department of Environment, Ministry of Energy, Science, Technology, Environment and Climate Change (MESTECC): Putrajaya, Malaysia, 2019.
- Wan Norhana, M.N.; Yurimoto, T.; Nurlemsha, B.I.; Roziawati, M.R.; Saadon, K. Food safety aspects in blood cockles (Tegillarca granosa) cultured off Selangor, Peninsular Malaysia. Malays. J. Sci. 2016, 35, 226–240. [Google Scholar] [CrossRef] [Green Version]
- Tavakoly Sany, S.B.; Hashim, R.; Rezayi, M.; Salleh, A.; Rahman, M.A.; Safari, O.; Sasekumar, A. Human health risk of polycyclic aromatic hydrocarbons from consumption of blood cockle and exposure to contaminated sediments and water along the Klang Strait, Malaysia. Mar. Pollut. Bull. 2014, 84, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Gaiero, D.M.; Ross, G.R.; Depetris, P.J.; Kempe, S. Spatial and Temporal Variability of Total Non-Residual Heavy Metals Content in Stream Sediments from the Suquia River System, Cordoba, Argentina. Water Air Soil Pollut. 1997, 93, 303–319. [Google Scholar] [CrossRef]
- Khan, M.Y.A.; Gani, K.M.; Chakrapani, G.J. Spatial and temporal variations of physicochemical and heavy metal pollution in Ramganga River—A tributary of River Ganges, India. Environ. Earth Sci. 2017, 76, 231. [Google Scholar] [CrossRef]
- Ishaque, A.B.; Johnson, L.; Gerald, T.; Boucaud, D.; Okoh, J.; Tchounwou, P.B. Assessment of Individual and Combined Toxicities of Four Non-Essential Metals (As, Cd, Hg and Pb) in the Microtox Assay. Int. J. Environ. Res. Public Health 2006, 3, 118–120. [Google Scholar] [CrossRef] [Green Version]
- Halmi, M.I.E.; Jirangon, H.; Johari, W.L.W.; Abdul Rachman, A.R.; Shukor, M.Y.; Syed, M.A. Comparison of Microtox and Xenoassay light as a near real time river monitoring assay for heavy metals. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Shukor, M.Y.A.; Baharom, N.A.; Rahman, F.A.; Abdullah, M.P.; Shamaan, N.A.; Syed, M.A. Development of a heavy metals enzymatic-based assay using papain. Anal. Chim. Acta 2006, 566, 283–289. [Google Scholar] [CrossRef]
- Shukor, M.Y.; Masdor, N.; Baharom, N.A.; Jamal, J.A.; Abdullah, M.P.A.; Shamaan, N.A.; Syed, M.A. An inhibitive determination method for heavy metals using bromelain, a cysteine protease. Appl. Biochem. Biotechnol. 2008, 144, 283–291. [Google Scholar] [CrossRef]
- Shukor, M.Y.; Baharom, N.A.; Masdor, N.A.; Abdullah, M.P.A.; Shamaan, N.A.; Jamal, J.A.; Syed, M.A. The development of an inhibitive determination method for zinc using a serine protease. J. Environ. Biol. 2009, 30, 17–22. [Google Scholar]
- Shukor, M.Y.; Tham, L.G.; Halmi, M.I.E.; Khalid, I.; Begum, G.; Syed, M.A. Development of an inhibitive assay using commercial Electrophorus electricus acetylcholinesterase for heavy metal detection. J. Environ. Biol. 2013, 34, 967–970. [Google Scholar]
- Shukor, M.Y.; Anuar, N.; Halmi, M.I.E.; Masdor, N.A. Near real-time inhibitive assay for heavy metals using achromopeptidase. Indian J. Biotechnol. 2014, 13, 398–403. [Google Scholar]
- Halmi, M.I.E.; Khayat, M.E.; Gunasekaran, B.; Masdor, N.A.; Rahman, M.F.A. Near real-time biomonitoring of copper from an industrial complex effluent discharge site using a plant protease inhibitive assay. Bioremediat. Sci. Technol. Res. 2016, 4, 10–13. [Google Scholar]
- Sabullah, M.K.; Sulaiman, M.R.; Shukor, M.S.; Yusof, M.T.; Johari, W.L.W.; Shukor, M.Y.; Syahir, A. Heavy metals biomonitoring via inhibitive assay of acetylcholinesterase from Periophthalmodon schlosseri. Rend. Lincei 2015, 26, 151–158. [Google Scholar] [CrossRef]
- Effendi Halmi, M.; Gunasekaran, B.; Shukor, M. An inhibitive determination method for biomonitoring of heavy metals using ficin; a cysteine plant protease. In Proceedings of the International Agriculture Congress, Putrajaya, Malaysia, 4–6 October 2016. [Google Scholar]
- Aziz, N.F.; Halmi, M.I.E.; Johari, W.L.W. Statistical optimization of hexavalent molybdenum reduction by Serratia sp. strain MIE2 using Central Composite Design (CCD). J. Biochem. Microbiol. Biotechnol. 2017, 5, 8–11. [Google Scholar]
- Roslan, M.A.H.; Abdullah, N.; Mustafa, S. Removal of shells in palm kernel cake via static cling and electrostatic separation. J. Biochem. Microbiol. Biotechnol. 2015, 3, 1–6. [Google Scholar]
- Bloomfield, M.S. A sensitive and rapid assay for 4-aminophenol in paracetamol drug and tablet formulation, by flow injection analysis with spectrophotometric detection. Talanta 2002, 58, 1301–1310. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Bozorgzadeh, E.; Shariati, S.; Zanjanchi, M.A. Trace determination of linear alkylbenzene sulfonates using ionic liquid based ultrasound-assisted dispersive liquid–liquid microextraction and response surface methodology. Anal. Methods 2012, 4, 2272–2277. [Google Scholar] [CrossRef]
- Shukor, M.; Masdor, N.; Halmi, M.; Kamaruddin, K.; Syed, M. Near-real-time biomonitoring of heavy metals using the xenoassay® system. In Proceedings of the 3rd Syiah Kuala University Annual International Conference 2013 (AIC Unsyiah), Syiah Kuala University—Life Sciences & Engineering Chapter, Banda Aceh, Indonesia, 2–4 October 2013; Volume 3. [Google Scholar]
- Bickerstaff, G.F.; Zhou, H. Protease activity and autodigestion (autolysis) assays using Coomassie blue dye binding. Anal. Biochem. 1993, 210, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, Y.; Yang, C.; Zeng, G.; Long, Z.; Wei, S.; Zhao, K.; Luo, L. Oxidative desulfurization of dibenzothiophene using a catalyst of molybdenum supported on modified medicinal stone. RSC Adv. 2016, 6, 17036–17045. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, K.; Cheng, Y.; Zeng, G.; Zhang, M.; Shao, J.; Lu, L. Catalytic oxidative desulfurization of BT and DBT from n-octane using cyclohexanone peroxide and catalyst of molybdenum supported on 4A molecular sieve. Sep. Purif. Technol. 2016, 163, 153–161. [Google Scholar] [CrossRef]
- Wei, S.; He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Kang, L.; Qian, H.; Zhu, C. Preparation, characterization, and catalytic performances of cobalt catalysts supported on KIT-6 silicas in oxidative desulfurization of dibenzothiophene. Fuel 2017, 200, 11–21. [Google Scholar] [CrossRef]
- Karamba, K.I.; Ahmad, S.A.; Zulkharnain, A.; Syed, M.A.; Khalil, K.A.; Shamaan, N.A.; Dahalan, F.A.; Shukor, M.Y. Optimisation of biodegradation conditions for cyanide removal by Serratia marcescens strain AQ07 using one-factor-at-a-time technique and response surface methodology. Rend. Lincei 2016, 27, 533–545. [Google Scholar] [CrossRef]
- Yakasai, M.H.; Rahman, M.F.A.; Rahim, M.B.H.A.; Khayat, M.E.; Shamaan, N.A.; Shukor, M.Y. Isolation and characterization of a metal-reducing Pseudomonas sp. strain 135 with amide-degrading capability. Bioremediat. Sci. Technol. Res. 2017, 5, 32–38. [Google Scholar]
- Islahuddin, N.K.S.; Halmi, M.I.E.; Manogaran, M.; Shukor, M.Y. Isolation and culture medium optimisation using one-factor-at-time and Response Surface Methodology on the biodegradation of the azo-dye amaranth. Bioremediat. Sci. Technol. Res. 2017, 5, 25–31. [Google Scholar]
- Whitcomb, P.J.; Anderson, M.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments; Productivity Press: New York, NY, USA, 2004; ISBN 978-1-56327-297-4. [Google Scholar]
- Antony, J. Design of Experiments for Engineers and Scientists, 1st ed.; Butterworth Heinemann: Oxford, UK; Burlinton, MA, USA, 2003; ISBN 978-0-7506-4709-0. [Google Scholar]
- Rosa, P.A.J.; Azevedo, A.M.; Aires-Barros, M.R. Application of central composite design to the optimisation of aqueous two-phase extraction of human antibodies. J. Chromatogr. A 2007, 1141, 50–60. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Hyne, R.V.; Pablo, F.; Day, W.R.; Doble, P. Optimisation of the separation of herbicides by linear gradient high performance liquid chromatography utilising artificial neural networks. Talanta 2007, 71, 1268–1275. [Google Scholar] [CrossRef]
- Marini, F.; Bucci, R.; Magrì, A.L.; Magrì, A.D. Artificial neural networks in chemometrics: History, examples and perspectives. Microchem. J. 2008, 88, 178–185. [Google Scholar] [CrossRef]
- Gunasekaran, B.; Ahmad, S.A.; Halmi, M.I.E. A rapid inhibitive enzyme assay for monitoring heavy metals pollution in a river running through a UNESCO heritage site. J. Biochem. Microbiol. Biotechnol. 2015, 3, 21–25. [Google Scholar]
- Hsieh, C.-Y.; Tsai, M.-H.; Ryan, D.K.; Pancorbo, O.C. Toxicity of the 13 priority pollutant metals to Vibrio fisheri in the Microtox® chronic toxicity test. Sci. Total Environ. 2004, 320, 37–50. [Google Scholar] [CrossRef]
- Aidil, M.S.; Sabullah, M.K.; Halmi, M.I.E.; Sulaiman, R.; Shukor, M.S.; Shukor, M.Y.; Shaharuddin, N.A.; Syed, M.A.; Syahir, A. Assay for heavy metals using an inhibitive assay based on the acetylcholinesterase from Pangasius hypophthalmus (Sauvage, 1878). Fresenius Environ. Bull. 2013, 22, 3572–3576. [Google Scholar]
- Halmi, M.I.E.; Johari, W.L.W.; Amir, S.; Sulaiman, R.; Azlina, A.; Shukor, M.Y.; Syed, M.A. Monitoring of heavy metals level in fish using Photobacterium sp. strain MIE. Bioremediat. Sci. Technol. Res. 2013, 1, 19–22. [Google Scholar]
- ELTurk, M.; Abdullah, R.; Mohamad Zakaria, R.; Abu Bakar, N.K. Heavy metal contamination in mangrove sediments in Klang estuary, Malaysia: Implication of risk assessment. Estuar. Coast. Shelf Sci. 2019, 226, 106266. [Google Scholar] [CrossRef]
- Wicke, D.; O’Sullivan, A.D.; Cochrane, T.A. Environmental CSI of the Okeover Stream in Christchurch; University of Canterbury, Civil and Natural Resources Engineering: Christchurch, New Zealand, 2009. [Google Scholar]
- Birch, G.F.; Taylor, S.E.; Matthai, C. Small-scale spatial and temporal variance in the concentration of heavy metals in aquatic sediments: A review and some new concepts. Environ. Pollut. 2001, 113, 357–372. [Google Scholar] [CrossRef]
- Jung, K.; Bitton, G.; Koopman, B. Assessment of urease inhibition assays for measuring toxicity of environmental samples. Water Res. 1995, 29, 1929–1933. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Singh, K.; Vig, A.P. Genotoxicity monitoring of industrial wastes using plant bioassays and management through vermitechnology: A review. Agric. Nat. Res. 2017, 51, 325–337. [Google Scholar] [CrossRef]
- Eom, H.; Ashun, E.; Toor, U.A.; Oh, S.-E. A solid-phase direct contact bioassay using sulfur-oxidizing bacteria (SOB) to evaluate toxicity of soil contaminated with heavy metals. Sens. Actuators B Chem. 2020, 305, 127510. [Google Scholar] [CrossRef]
- Polyakova, G.; Pashenova, N.; Senashova, V.; Podolyak, N.; Kudryasheva, N. Pine Stands as Bioindicators: Justification for Air Toxicity Monitoring in an Industrial Metropolis. Environments 2020, 7, 28. [Google Scholar] [CrossRef]






| Figure | Name | Units | Type | Low Actual | High Actual | Low Coded | High Coded |
|---|---|---|---|---|---|---|---|
| A | pH | min | Numeric | 5.8 | 7.8 | −1 | 1 |
| B | Ficin | mg/mL | Numeric | 0.1 | 1 | −1 | 1 |
| C | Casein | mg/mL | Numeric | 1 | 4 | −1 | 1 |
| Run. | pH | Ficin (mg/mL) | Casein (mg/mL) | Response (Abs595 nm) | Predicted (Abs595 nm) |
|---|---|---|---|---|---|
| 1 | 6.8 | 0.55 | 2.5 | 0.201 | 0.200 |
| 2 | 6.8 | 0.55 | 5.02 | 0.071 | 0.071 |
| 3 | 7.8 | 1 | 1 | 0.061 | 0.059 |
| 4 | 7.8 | 0.1 | 1 | 0.041 | 0.045 |
| 5 | 7.8 | 1 | 4 | 0.048 | 0.051 |
| 6 | 6.8 | 0.55 | 2.5 | 0.201 | 0.200 |
| 7 | 6.8 | 0.55 | 2.5 | 0.205 | 0.200 |
| 8 | 5.8 | 1 | 1 | 0.038 | 0.040 |
| 9 | 6.8 | 0.55 | 2.5 | 0.197 | 0.200 |
| 10 | 6.8 | 0.55 | 2.5 | 0.205 | 0.200 |
| 11 | 8.48 | 0.55 | 2.5 | 0.031 | 0.029 |
| 12 | 5.8 | 0.1 | 4 | 0.041 | 0.044 |
| 13 | 6.8 | 0.55 | 2.5 | 0.205 | 0.200 |
| 14 | 5.8 | 0.1 | 1 | 0.068 | 0.066 |
| 15 | 6.8 | 0.21 | 2.5 | 0.032 | 0.031 |
| 16 | 6.8 | 1.31 | 2.5 | 0.048 | 0.049 |
| 17 | 7.8 | 0.1 | 4 | 0.006 | 0.004 |
| 18 | 5.8 | 1 | 4 | 0.055 | 0.051 |
| 19 | 5.12 | 0.55 | 2.5 | 0.045 | 0.046 |
| 20 | 6.8 | 0.55 | −0.02 | 0.098 | 0.097 |
| Sum of | Mean | F | ||||
|---|---|---|---|---|---|---|
| Source | Squares | DF | Square | Value | Prob > F | |
| Model | 0.105101 | 9 | 0.011678 | 899.0521 | <0.0001 | significant |
| A | 0.000354 | 1 | 0.000354 | 27.26442 | 0.0004 | |
| B | 0.000389 | 1 | 0.000389 | 29.95455 | 0.0003 | |
| C | 0.000783 | 1 | 0.000783 | 60.27813 | <0.0001 | |
| A2 | 0.048926 | 1 | 0.048926 | 3766.679 | <0.0001 | |
| B2 | 0.047597 | 1 | 0.047597 | 3664.359 | <0.0001 | |
| C2 | 0.025238 | 1 | 0.025238 | 1943.044 | <0.0001 | |
| AB | 0.000761 | 1 | 0.000761 | 58.54923 | <0.0001 | |
| AC | 0.000181 | 1 | 0.000181 | 13.8963 | 0.0039 | |
| BC | 0.000544 | 1 | 0.000544 | 41.91986 | <0.0001 | |
| Residual | 0.00013 | 10 | 1.3 × 10−5 | |||
| Lack of Fit | 7.66 × 10−5 | 5 | 1.53 × 10−5 | 1.435451 | 0.3506 | not significant |
| Pure Error | 5.33 × 10−5 | 5 | 1.07 × 10−5 | |||
| Cor Total | 0.105231 | 19 |
| OFAT | RSM | |||
|---|---|---|---|---|
| Metals | LOD (mg/L) | IC50 (mg/L) | LOD (mg/L) | IC50 (mg/L) |
| Hg2+ | 0.018 (0.013 to 0.023) | 0.060 (0.030 to 0.080) | 0.002 (0.001 to 0.004) | 0.017 (0.016 to 0.019) |
| Ag+ | 0.010 (0.007 to 0.018) | 0.098 (0.077 to 0.127) | 0.003 (0.002 to 0.004) | 0.028 (0.022 to 0.037) |
| Cu2+ | 0.019 (0.016 to 0.021) | 0.040 (0.035 to 0.045) | 0.002 (0.001 to 0.004) | 0.023 (0.020 to 0.027) |
| LC50 or IC50 (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Metals | 15-min. MicrotoxTM a, c | 48 h Daphnia magna a | 96 h Rainbow trout a c | Papain b | Bromelain d | AChE from Pangasius sp. d | This Study | Mo-Reducing Enzyme from Serratia sp. Strain DrY8 e |
| Cu2+ Hg2+ Ag+ | 0.076–3.8 0.029–0.050 n.i. | 0.020–0.093 0.005–0.21 1.930 | 0.25 0.033–0.210 0.050 | 0.004 0.24–0.62 0.33–0.49 | 0.163–0.305 0.13–0.16 n.i. | 0.065–0.096 0.059–0.088 0.082–0.095 | 0.020–0.027 0.016–0.019 0.022–0.037 | 0.295–0.435 0.154–0.178 0.018–0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uba, G.; Manogaran, M.; Gunasekaran, B.; Halmi, M.I.E.; Shukor, M.Y.A. Improvement of Ficin-Based Inhibitive Enzyme Assay for Toxic Metals Using Response Surface Methodology and Its Application for Near Real-Time Monitoring of Mercury in Marine Waters. Int. J. Environ. Res. Public Health 2020, 17, 8585. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228585
Uba G, Manogaran M, Gunasekaran B, Halmi MIE, Shukor MYA. Improvement of Ficin-Based Inhibitive Enzyme Assay for Toxic Metals Using Response Surface Methodology and Its Application for Near Real-Time Monitoring of Mercury in Marine Waters. International Journal of Environmental Research and Public Health. 2020; 17(22):8585. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228585
Chicago/Turabian StyleUba, Garba, Motharasan Manogaran, Baskaran Gunasekaran, Mohd Izuan Effendi Halmi, and Mohd Yunus Abd Shukor. 2020. "Improvement of Ficin-Based Inhibitive Enzyme Assay for Toxic Metals Using Response Surface Methodology and Its Application for Near Real-Time Monitoring of Mercury in Marine Waters" International Journal of Environmental Research and Public Health 17, no. 22: 8585. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228585






