Cardiorespiratory Fitness Mediates Cognitive Performance in Chronic Heart Failure Patients and Heart Transplant Recipients
Abstract
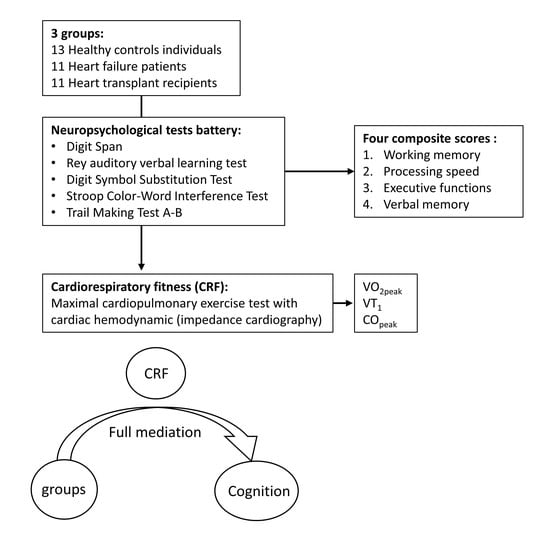
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Maximal Cardiopulmonary Exercise Test (CPET)
2.4. Cognitive Evaluation
2.5. Statistical Analysis
3. Results
3.1. Group Comparisons
3.2. Univariate Correlations
3.3. Mediation Analyses
4. Discussion
4.1. Cognition in Chronic Heart Failure and in Heart Transplant Recipients
4.2. Related Mechanisms of Cognitive Impairment
4.3. Cardiorespiratory Fitness in Chronic Heart Failure and in Heart Transplant Recipients
4.4. Relationship between O2peak, COpeak and Cognitive Performance
4.5. Study limitations and Research Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cannon, J.A.; Moffitt, P.; Perez-Moreno, A.C.; Walters, M.R.; Broomfield, N.M.; McMurray, J.J.V.; Quinn, T.J. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J. Card. Fail. 2017, 23, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Zuccala, G.; Pedone, C.; Cesari, M.; Onder, G.; Pahor, M.; Marzetti, E.; Lo Monaco, M.R.; Cocchi, A.; Carbonin, P.; Bernabei, R. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am. J. Med. 2003, 115, 97–103. [Google Scholar] [CrossRef]
- Cameron, J.; Gallagher, R.; Pressler, S.J. Detecting and Managing Cognitive Impairment to Improve Engagement in Heart Failure Self-Care. Curr. Heart Fail. Rep. 2017, 14, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.S.; Kazim, R.; Xu, J.; Borson, S.; Taffet, G.E. Unrecognized Cognitive Impairment and Its Effect on Heart Failure Readmissions of Elderly Adults. J. Am. Geriatr. Soc. 2016, 64, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014, 171, 368–376. [Google Scholar] [CrossRef]
- Trojano, L.; Antonelli Incalzi, R.; Acanfora, D.; Picone, C.; Mecocci, P.; Rengo, F.; Congestive Heart Failure Italian Study Investigators. Cognitive impairment: A key feature of congestive heart failure in the elderly. J. Neurol. 2003, 250, 1456–1463. [Google Scholar] [CrossRef]
- Abete, P.; Della-Morte, D.; Gargiulo, G.; Basile, C.; Langellotto, A.; Galizia, G.; Testa, G.; Canonico, V.; Bonaduce, D.; Cacciatore, F. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res. Rev. 2014, 18, 41–52. [Google Scholar] [CrossRef]
- Deshields, T.L.; McDonough, E.M.; Mannen, R.K.; Miller, L.W. Psychological and cognitive status before and after heart transplantation. Gen. Hosp. Psychiatry 1996, 18, 62S–69S. [Google Scholar] [CrossRef]
- Bornstein, R.A.; Starling, R.C.; Myerowitz, P.D.; Haas, G.J. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol. Scand. 1995, 91, 260–265. [Google Scholar] [CrossRef]
- Schall, R.R.; Petrucci, R.J.; Brozena, S.C.; Cavarocchi, N.C.; Jessup, M. Cognitive function in patients with symptomatic dilated cardiomyopathy before and after cardiac transplantation. J. Am. Coll Cardiol. 1989, 14, 1666–1672. [Google Scholar] [CrossRef] [Green Version]
- Roman, D.D.; Kubo, S.H.; Ormaza, S.; Francis, G.S.; Bank, A.J.; Shumway, S.J. Memory improvement following cardiac transplantation. J. Clin. Exp. Neuropsychol. 1997, 19, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Cupples, S.A.; Stilley, C.S. Cognitive function in adult cardiothoracic transplant candidates and recipients. J. Cardiovasc. Nurs. 2005, 20, S74–S87. [Google Scholar] [CrossRef] [PubMed]
- Burker, B.S.; Gude, E.; Gullestad, L.; Grov, I.; Relbo Authen, A.; Andreassen, A.K.; Havik, O.E.; Dew, M.A.; Fiane, A.E.; Haraldsen, I.R.; et al. Cognitive function among long-term survivors of heart transplantation. Clin. Transpl. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Spitznagel, M.B.; van Dulmen, M.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; et al. Cognitive function and treatment adherence in older adults with heart failure. Psychosom. Med. 2012, 74, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, M.H.; Hogan, D.B.; Eskes, G.A.; Longman, R.S.; Poulin, M.J. Cerebrovascular reserve: The link between fitness and cognitive function? Exerc. Sport Sci. Rev. 2012, 40, 153–158. [Google Scholar] [CrossRef]
- Hajduk, A.M.; Kiefe, C.I.; Person, S.D.; Gore, J.G.; Saczynski, J.S. Cognitive change in heart failure: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Putzke, J.D.; Williams, M.A.; Rayburn, B.K.; Kirklin, J.K.; Boll, T.J. The relationship between cardiac function and neuropsychological status among heart transplant candidates. J. Card. Fail. 1998, 4, 295–303. [Google Scholar] [CrossRef]
- Gayda, M.; Desjardins, A.; Lapierre, G.; Dupuy, O.; Fraser, S.; Bherer, L.; Juneau, M.; White, M.; Gremeaux, V.; Labelle, V.; et al. Cerebral Hemodynamics During Exercise and Recovery in Heart Transplant Recipients. Can. J. Cardiol. 2016, 32, 539–546. [Google Scholar] [CrossRef]
- Gayda, M.; Gremeaux, V.; Bherer, L.; Juneau, M.; Drigny, J.; Dupuy, O.; Lapierre, G.; Labelle, V.; Fortier, A.; Nigam, A. Cognitive function in patients with stable coronary heart disease: Related cerebrovascular and cardiovascular responses. PLoS ONE 2017, 12, e0183791. [Google Scholar] [CrossRef] [Green Version]
- Putzke, J.D.; Williams, M.A.; Daniel, F.J.; Bourge, R.C.; Boll, T.J. Activities of daily living among heart transplant candidates: Neuropsychological and cardiac function predictors. J. Heart Lung Transpl. 2000, 19, 995–1006. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Sweet, L.H.; Hayes, S.M.; Josephson, R.; Hughes, J.; Gunstad, J. Decreases in daily physical activity predict acute decline in attention and executive function in heart failure. J. Card. Fail. 2015, 21, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alosco, M.L.; Penn, M.S.; Spitznagel, M.B.; Cleveland, M.J.; Ott, B.R.; Gunstad, J. Reduced Physical Fitness in Patients with Heart Failure as a Possible Risk Factor for Impaired Driving Performance. Am. J. Occup. 2015, 69, 6902260010p1–6902260010p8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alosco, M.L.; Spitznagel, M.B.; Sweet, L.H.; Josephson, R.; Hughes, J.; Gunstad, J. Cognitive dysfunction mediates the effects of poor physical fitness on decreased functional independence in heart failure. Geriatr. Gerontol. Int. 2015, 15, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Langeard, A.; Kaushal, N.; Vrinceanu, T.; Desjardins-Crepeau, L.; Langlois, F.; Kramer, A.F. Physical exercise training effect and mediation through cardiorespiratory fitness on dual-task performances differ in younger-old and older-old adults. J. Gerontol B Psychol. Sci. Soc. Sci. 2019. [Google Scholar] [CrossRef]
- Gayda, M.; Normandin, E.; Meyer, P.; Juneau, M.; Haykowsky, M.; Nigam, A. Central hemodynamic responses during acute high-intensity interval exercise and moderate continuous exercise in patients with heart failure. Appl. Physiol. Nutr. Metab. 2012, 37, 1171–1178. [Google Scholar] [CrossRef]
- Lezak, M.D. Neuropsychological Assessment; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Desjardins-Crepeau, L.; Berryman, N.; Vu, T.T.; Villalpando, J.M.; Kergoat, M.J.; Li, K.Z.; Bosquet, L.; Bherer, L. Physical functioning is associated with processing speed and executive functions in community-dwelling older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, 837–844. [Google Scholar] [CrossRef]
- Hayes, A.F.; Preacher, K.J. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math Stat. Psychol. 2014, 67, 451–470. [Google Scholar] [CrossRef]
- Grimm, M.; Yeganehfar, W.; Laufer, G.; Madl, C.; Kramer, L.; Eisenhuber, E.; Simon, P.; Kupilik, N.; Schreiner, W.; Pacher, R.; et al. Cyclosporine may affect improvement of cognitive brain function after successful cardiac transplantation. Circulation 1996, 94, 1339–1345. [Google Scholar] [CrossRef]
- Jha, S.R.; Hannu, M.K.; Chang, S.; Montgomery, E.; Harkess, M.; Wilhelm, K.; Hayward, C.S.; Jabbour, A.; Spratt, P.M.; Newton, P.; et al. The Prevalence and Prognostic Significance of Frailty in Patients with Advanced Heart Failure Referred for Heart Transplantation. Transplantation 2016, 100, 429–436. [Google Scholar] [CrossRef]
- Mapelli, D.; Bardi, L.; Mojoli, M.; Volpe, B.; Gerosa, G.; Amodio, P.; Daliento, L. Neuropsychological profile in a large group of heart transplant candidates. PLoS ONE 2011, 6, e28313. [Google Scholar] [CrossRef] [Green Version]
- Roman, D.D.; Holker, E.G.; Missov, E.; Colvin, M.M.; Menk, J. Neuropsychological functioning in heart transplant candidates. Clin. Neuropsychol. 2017, 31, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Putzke, J.D.; Williams, M.A.; Daniel, J.F.; Foley, B.A.; Kirklin, J.K.; Boll, T.J. Neuropsychological functioning among heart transplant candidates: A case control study. J. Clin. Exp. Neuropsychol. 2000, 22, 95–103. [Google Scholar] [CrossRef]
- Kanbay, M.; Sanchez-Lozada, L.G.; Franco, M.; Madero, M.; Solak, Y.; Rodriguez-Iturbe, B.; Covic, A.; Johnson, R.J. Microvascular disease and its role in the brain and cardiovascular system: A potential role for uric acid as a cardiorenal toxin. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillard, T. Preventive effects of regular physical exercise against cognitive decline and the risk of dementia with age advancement. Sports Med. Open 2015, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, E.P.; O’Dwyer, N.; Cook, R.; Vetter, M.; Cheng, H.L.; Rooney, K.; O’Connor, H. Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: A systematic review. J. Sci. Med. Sport Sports Med. Aust. 2015. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Jichici, D.; Petrucci, R.; Urrutia, V.C.; Schwartzman, R.J. Neurologic complications of the Novacor left ventricular assist device. Ann. Thorac. Surg. 2001, 72, 1311–1315. [Google Scholar] [CrossRef]
- Gary, R.A.; Brunn, K. Aerobic exercise as an adjunct therapy for improving cognitive function in heart failure. Cardiol. Res. Pract. 2014, 2014, 157508. [Google Scholar] [CrossRef]
- Ogren, J.A.; Fonarow, G.C.; Woo, M.A. Cerebral impairment in heart failure. Curr. Heart Fail. Rep. 2014, 11, 321–329. [Google Scholar] [CrossRef]
- Almeida, O.P.; Garrido, G.J.; Beer, C.; Lautenschlager, N.T.; Arnolda, L.; Flicker, L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur. Heart J. 2012, 33, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.; Christle, J.W.; Tun, A.; Yilmaz, B.; Moneghetti, K.J.; Yuen, E.; Soofi, M.; Ashley, E. Cardiopulmonary Exercise Testing, Impedance Cardiography, and Reclassification of Risk in Patients Referred for Heart Failure Evaluation. J. Card. Fail. 2019, 25, 961–968. [Google Scholar] [CrossRef]
- Conraads, V.M.; Van Craenenbroeck, E.M.; De Maeyer, C.; Van Berendoncks, A.M.; Beckers, P.J.; Vrints, C.J. Unraveling new mechanisms of exercise intolerance in chronic heart failure: Role of exercise training. Heart Fail. Rev. 2013, 18, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Beaudry, R.I.; Samuel, T.J.; Nelson, M.D.; Halle, M.; Baggish, A.L.; Haykowsky, M.J. Performance Limitations in Heart Transplant Recipients. Exerc. Sport Sci. Rev. 2018, 46, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Yardley, M.; Havik, O.E.; Grov, I.; Relbo, A.; Gullestad, L.; Nytroen, K. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin. Transpl. 2016, 30, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mezzani, A.; Hamm, L.F.; Jones, A.M.; McBride, P.E.; Moholdt, T.; Stone, J.A.; Urhausen, A.; Williams, M.A. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, 442–467. [Google Scholar] [CrossRef]
- Temple, R.O.; Putzke, J.D.; Boll, T.J. Neuropsychological performance as a function of cardiac status among heart transplant candidates: A replication. Percept. Mot. Ski. 2000, 91, 821–825. [Google Scholar] [CrossRef]
- Kelly, M.E.; Loughrey, D.; Lawlor, B.A.; Robertson, I.H.; Walsh, C.; Brennan, S. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2014, 16, 12–31. [Google Scholar] [CrossRef]
- Sexton, C.E.; Betts, J.F.; Demnitz, N.; Dawes, H.; Ebmeier, K.P.; Johansen-Berg, H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage 2015. [Google Scholar] [CrossRef] [Green Version]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef] [Green Version]
- Caldas, J.R.; Panerai, R.B.; Haunton, V.J.; Almeida, J.P.; Ferreira, G.S.; Camara, L.; Nogueira, R.C.; Bor-Seng-Shu, E.; Oliveira, M.L.; Groehs, R.R.; et al. Cerebral blood flow autoregulation in ischemic heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R108–R113. [Google Scholar] [CrossRef] [Green Version]
- Smirl, J.D.; Haykowsky, M.J.; Nelson, M.D.; Tzeng, Y.C.; Marsden, K.R.; Jones, H.; Ainslie, P.N. Relationship between cerebral blood flow and blood pressure in long-term heart transplant recipients. Hypertension 2014, 64, 1314–1320. [Google Scholar] [CrossRef]
- Massaro, A.R.; Dutra, A.P.; Almeida, D.R.; Diniz, R.V.; Malheiros, S.M. Transcranial Doppler assessment of cerebral blood flow: Effect of cardiac transplantation. Neurology 2006, 66, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Nobre, T.S.; Antunes-Correa, L.M.; Groehs, R.V.; Alves, M.J.; Sarmento, A.O.; Bacurau, A.V.; Urias, U.; Alves, G.B.; Rondon, M.U.; Brum, P.C.; et al. Exercise training improves neurovascular control and calcium cycling gene expression in patients with heart failure with cardiac resynchronization therapy. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1180–H1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, T.C.; Wang, C.H.; Lin, P.S.; Hsu, C.C.; Cherng, W.J.; Huang, S.C.; Liu, M.H.; Chiang, C.L.; Wang, J.S. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int. J. Cardiol. 2013, 167, 41–50. [Google Scholar] [CrossRef]
- Chen, F.T.; Hopman, R.J.; Huang, C.J.; Chu, C.H.; Hillman, C.H.; Hung, T.M.; Chang, Y.K. The Effect of Exercise Training on Brain Structure and Function in Older Adults: A Systematic Review Based on Evidence from Randomized Control Trials. J. Clin. Med. 2020, 9, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, F.; Muller, P.; Gronwald, T.; Muller, N.G. Dose-Response Matters! A Perspective on the Exercise Prescription in Exercise-Cognition Research. Front. Psychol. 2019, 10, 2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Cardiac rehabilitation is associated with lasting improvements in cognitive function in older adults with heart failure. Acta Cardiol. 2014, 69, 407–414. [Google Scholar] [CrossRef]
- Tanne, D.; Freimark, D.; Poreh, A.; Merzeliak, O.; Bruck, B.; Schwammenthal, Y.; Schwammenthal, E.; Motro, M.; Adler, Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int. J. Cardiol. 2005, 103, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Res. Rev. 2020, 60, 101044. [Google Scholar] [CrossRef]
- Smirl, J.D.; Haykowsky, M.J.; Nelson, M.D.; Altamirano-Diaz, L.A.; Ainslie, P.N. Resting and exercise cerebral blood flow in long-term heart transplant recipients. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2012, 31, 906–908. [Google Scholar] [CrossRef]
- Rouch, L.; Cestac, P.; Hanon, O.; Cool, C.; Helmer, C.; Bouhanick, B.; Chamontin, B.; Dartigues, J.F.; Vellas, B.; Andrieu, S. Antihypertensive drugs, prevention of cognitive decline and dementia: A systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs 2015, 29, 113–130. [Google Scholar] [CrossRef]
- Lee, J.K.; Son, Y.J. Gender Differences in the Impact of Cognitive Function on Health Literacy among Older Adults with Heart Failure. Int. J. Environ. Res. Public Health 2018, 15, 2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| HF n = 11 | HT n = 11 | HC n = 13 | p Value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (years) | 66.18 ± 7.88 | 58.55 ± 7.92 | 60.15 ± 8.49 | 0.081 |
| Sex (F/M) | 1/10 | 1/10 | 1/12 | - |
| Heigh (cm) | 172.45 ± 8.26 | 169.73 ± 7.36 | 172.69 ± 8.28 | 0.621 |
| Body mass (kg) | 77.38 ± 10.91 | 78.07 ± 15.85 | 73.53 ± 8.09 | 0.532 |
| BMI (kg/m2) | 25.97 ± 2.89 | 27.04 ± 4.95 | 24.65 ± 2.03 | 0.236 |
| Body surface (m2) | 1.92 ± 0.17 | 1.91 ± 0.21 | 1.88 ± 0.14 | 0.796 |
| Level of education (years) | 14.36 ± 3.83 | 11.82 ± 3.09 | 14.67 ± 2.67 | 0.086 |
| Time from transplantation (years) | - | 7.40 ± 5.40 | - | - |
| Medication | ||||
| Immunosuppressive drugs * | 11 (100%) | |||
| Beta blockers | 11 (100%) | 3 (27%) | ||
| ACE inhibitors | 6 (55%) | 0 | ||
| ARBs | 6 (55%) | 6 (55%) | ||
| Diuretics | 9 (82%) |
| Cardiopulmonary and Hemodynamic Variables | HF n = 11 | HT n = 11 | HC n = 13 | p Value | HF vs. HT | HF vs. HC | HT vs. HC |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Peak power output (watts) | 87.27 ± 26.96 | 134.09 ± 66.96 | 225.77 ± 54.42 | <0.0001 | 0.051 | <0.0001 | 0.002 |
| O2peak (mL/min) | 1346 ± 316 | 1981 ± 626 | 2780 ± 606 | <0.0001 | 0.009 | <0.0001 | 0.005 |
| O2peak (mL/min/kg) | 17.60 ± 4.31 | 26.46 ± 10.12 | 37.69 ± 7.27 | <0.0001 | 0.019 | <0.0001 | 0.007 |
| RER | 1.14 ± 0.09 | 1.12 ± 0.09 | 1.16 ± 0.08 | 0.480 | - | - | - |
| HRpeak (bpm) | 113.91 ± 20.81 | 138.27 ± 21.28 | 157.15 ± 11.47 | <0.0001 | 0.013 | <0.0001 | 0.019 |
| O2 pulse (mLO2/bpm) | 12.02 ± 2.40 | 14.17 ± 3.15 | 17.70 ± 3.72 | 0.001 | 0.122 | <0.001 | 0.011 |
| SBPpeak (mmHg) | 141.4 ± 19.7 | 176.8 ± 22.6 | 184.8 ± 23.8 | <0.0001 | 0.001 | <0.0001 | 0.384 |
| DBPpeak (mmHg) | 68.5 ± 6.3 | 74.1 ± 7.7 | 78.6 ± 11.6 | 0.032 | 0.076 | 0.014 | 0.267 |
| CIpeak (L/min/m2) | 5.35 ± 1.50 | 6.97 ± 1.52 | 7.98 ± 1.05 | <0.001 | 0.008 | <0.0001 | 0.080 |
| COpeak (L/min) | 10.29 ± 2.89 | 13.11 ± 2.57 | 14.51 ± 2.13 | 0.001 | 0.013 | <0.001 | 0.185 |
| LCWipeak (kg.m/m2) | 6.50 ± 2.55 | 9.85 ± 2.42 | 11.38 ± 2.28 | <0.0001 | 0.003 | <0.0001 | 0.131 |
| O2 at VT1 (mL/min) | 918 ± 170 | 1309 ± 284 | 2108 ± 528 | <0.0001 | 0.002 | <0.0001 | <0.001 |
| Power at VT1 (watts) | 51.36 ± 17.62 | 69.90 ± 27.20 | 162.08 ± 47.79 | <0.0001 | 0.086 | <0.0001 | <0.0001 |
| HF n = 11 | HT n = 11 | HC n = 13 | p Value | HF vs. HT | HF vs. HC | HT vs. HC | |
|---|---|---|---|---|---|---|---|
| Neuropsychological Tests | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| MMSE | 26.82 ± 1.08 | 27.09 ± 2.47 | 28.85 ± 0.90 | <0.001 | 0.742 | <0.0001 | 0.045 |
| Geriatric Depression Scale | 9.18 ± 8.61 | 4.36 ± 2.25 | 3.33 ± 3.28 | 0.140 | - | - | - |
| Forward | 8.91 ± 2.39 | 9.55 ± 1.75 | 10.00 ± 1.21 | 0.396 | - | - | - |
| Backward | 6.45 ± 2.38 | 6.36 ± 1.75 | 7.17 ± 2.29 | 0.622 | - | - | - |
| DSST | 52.45 ± 12.14 | 51.64 ± 14.40 | 69.62 ± 12.15 | 0.002 | 0.883 | 0.003 | 0.002 |
| TMT A (s) | 47.53 ± 15.54 | 38.86 ± 10.81 | 36.34 ± 12.59 | 0.114 | - | - | - |
| TMT B (s) | 112.10 ± 54.53 | 85.21 ± 34.30 | 67.46 ± 17.98 | 0.043 | 0.190 | 0.024 | 0.161 |
| TMT (B-A)/A | 1.33 ± 0.92 | 1.16 ± 0.64 | 0.95 ± 0.44 | 0.397 | - | - | - |
| Stroop Test | |||||||
| Stroop 1 (s) | 31.19 ± 4.09 | 33.89 ± 5.67 | 29.41 ± 4.96 | 0.135 | - | - | - |
| Stroop 2 (s) | 22.63 ± 4.57 | 23.39 ± 4.33 | 20.10 ± 3.35 | 0.144 | - | - | - |
| Stroop 3 (s) | 72.39 ± 9.60 | 63.28 ± 9.19 | 52.05 ± 11.02 | <0.001 | 0.041 | <0.0001 | 0.011 |
| Stroop 4 (s) | 74.21 ± 13.51 | 73.22 ± 17.76 | 55.86 ± 16.18 | 0.014 | 0.884 | 0.010 | 0.014 |
| RAVL Test | |||||||
| Immediate recall | 6.55 ± 2.25 | 8.18 ± 4.29 | 10.38 ± 3.04 | 0.009 | 0.280 | 0.002 | 0.171 |
| Delayed recall | 6.40 ± 2.32 | 9.45 ± 3.42 | 10.00 ± 2.37 | 0.013 | 0.017 | 0.006 | 0.647 |
| Rey 1–5 total words | 35.18 ± 5.46 | 46.82 ± 8.65 | 51.62 ± 8.11 | <0.0001 | 0.001 | <0.0001 | 0.132 |
| Composite Z scores | |||||||
| Working memory | −0.21 ± 1.11 | −0.06 ± 0.82 | 0.25 ± 0.60 | 0.425 | - | - | - |
| Processing speed | 0.26 ± 0.61 | 0.28 ± 0.78 | −0.43 ± 0.73 | 0.029 | 0.958 | 0.024 | 0.021 |
| Executive functioning | 0.59 ± 0.63 | 0.14 ± 0.75 | −0.63 ± 0.60 | <0.001 | 0.120 | <0.0001 | 0.008 |
| Verbal memory | −0.78 ± 0.53 | 0.12 ± 0.99 | 0.56 ± 0.78 | 0.001 | 0.012 | <0.001 | 0.178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besnier, F.; Bérubé, B.; Gagnon, C.; Olmand, M.; Ribeiro, P.A.B.; Nigam, A.; Juneau, M.; Blondeau, L.; White, M.; Gremeaux, V.; et al. Cardiorespiratory Fitness Mediates Cognitive Performance in Chronic Heart Failure Patients and Heart Transplant Recipients. Int. J. Environ. Res. Public Health 2020, 17, 8591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228591
Besnier F, Bérubé B, Gagnon C, Olmand M, Ribeiro PAB, Nigam A, Juneau M, Blondeau L, White M, Gremeaux V, et al. Cardiorespiratory Fitness Mediates Cognitive Performance in Chronic Heart Failure Patients and Heart Transplant Recipients. International Journal of Environmental Research and Public Health. 2020; 17(22):8591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228591
Chicago/Turabian StyleBesnier, Florent, Béatrice Bérubé, Christine Gagnon, Miloudza Olmand, Paula Aver Bretanha Ribeiro, Anil Nigam, Martin Juneau, Lucie Blondeau, Michel White, Vincent Gremeaux, and et al. 2020. "Cardiorespiratory Fitness Mediates Cognitive Performance in Chronic Heart Failure Patients and Heart Transplant Recipients" International Journal of Environmental Research and Public Health 17, no. 22: 8591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228591