Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer
Abstract
:1. Introduction
- -
- Direct neurotoxic effects—cytostatic drugs that cross the blood–brain barrier can cause cell death [5].
- -
- Induced hormonal changes—these changes can interfere with hormone secretion and activate cognitive problems. It is known that chemotherapy changes the testosterone and estrogen levels, which are considered neuroprotective hormones.
- -
- Oxidative stress—chemotherapy decreases the cellular antioxidant capacity and thus increases DNA damage [6].
- -
- Immune system dysregulation caused by cytokine release—inflammatory cytokines cross the blood–brain barrier and can cause a decline in cognitive function, manifested as decreased processing speed, executive function, spatial ability, and reaction time [7].
- -
- Vascular damage—coagulation in small vessels of the central nervous system, vascular damage, and autoimmune phenomena. Both chemotherapy and radiotherapy can damage blood vessels, which reduce blood flow in the small blood vessels in the brain [8].
1.1. Causes that Speed up Impairment
- -
- Chemotherapy-related cognitive impairment—toxicity might produce cognitive changes when crossing the blood–brain barrier.
- -
- Stress-related cognitive impairment—stress can negatively affect memory as it increases cortisol released by the adrenal glands, and this substance directly affects the hippocampus, which is part of the limbic system dedicated to working and short-term memory [11].
- -
- Anxiety-related cognitive impairment—excessive worry and irrational fear can impair memory by focusing thought on a particular obsession. Memory suffers from anxiety and can cause memory loss [12].
- -
- Depression-related cognitive impairment—depression might be related to attentional problems, which affect the information acquisition and coding phase. The data provided by different scientific studies show adverse neuropsychological effects of chemotherapy [13].
1.2. Study Hypotheses and Objectives
- To analyze the impact of chemotherapy on the cognitive domains in the three study periods measured.
- To assess whether the emotional state affects the cognitive performance of patients.
- To define if there are changes in the quality of life of the patients during the treatment and if this affects their cognitive performance.
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.4. Description of the Variables
2.5. Evaluation Tools
- -
- Processing speed—measured by the Symbol Search and Key Search Subtests.
- -
- Attention—measured by the Trail Making Test and the Stroop Color and Word Test [30].
- -
- Memory—measured by the Vocabulary Subtest.
2.6. Statistical Analysis
2.7. Descriptive Statistics
2.8. Analytical Statistics
3. Results
- -
- Symbol Search before (F = 4.234; p < 0.05);
- -
- Letters and Number before (F = 5.152; p < 0.01);
- -
- Stroop Word before (F = 4.746; p < 0.001);
- -
- Stroop Color and Word before (F = 7.582; p < 0.01) and
- -
- Stroop Color and Word during (F = 7.102; p < 0.01)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurria, A.; Goldfarb, S.; Rosen, C.; Holland, J.; Zuckerman, E.; Lachs, M.S.; Witmer, M.; Van Gorp, W.G.; Fornier, M.; D’Andrea, G.; et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Res. Treat. 2006, 98, 343–348. [Google Scholar] [CrossRef]
- Hermelink, K.; Untch, M.; Lux, M.P.; Kreienberg, R.; Beck, T.; Bauerfeind, I.; Münzel, K. Cognitive function during neoadjuvant chemotherapy for breast cancer. Cancer 2007, 109, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Vardy, J.L.; Binns, M.; Kerr, L.; Tannock, I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol. Biochem. Behav. 2006, 85, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Vezmar, S.; Schüsseler, P.; Becker, A.; Bode, U.; Jaehde, U. Methotrexate-associated alterations of the folate and methyl-transfer pathway in the CSF of ALL patients with and without symptoms of neurotoxicity. Pediatr. Blood Cancer 2009, 52, 26–32. [Google Scholar] [CrossRef]
- Vardy, J.; Dhillon, H. The fog hasn’t lifted on “chemobrain” yet: Ongoing uncertainty regarding the effects of chemotherapy and breast cancer on cognition. Breast Cancer Res. Treat. 2010, 123, 35–37. [Google Scholar] [CrossRef]
- Hoag, H. The foggy world of chemobrain. Tor. Star 2007, 3, 13–16. [Google Scholar]
- Hurria, A.; Rosen, C.; Hudis, C.; Zuckerman, E.; Panageas, K.S.; Lachs, M.S.; Witmer, M.; van Gorp, W.G.; Fornier, M.; D’Andrea, G.; et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J. Am. Geriatr. Soc. 2006, 54, 925–931. [Google Scholar] [CrossRef]
- Deprez, S.; Amant, F.; Smeets, A.; Peeters, R.; Leemans, A.; Van Hecke, W.; Verhoeven, J.S.; Christiaens, M.-R.; Vandenberghe, J.; Vandenbulcke, M.; et al. Longitudinal Assessment of Chemotherapy-Induced Structural Changes in Cerebral White Matter and Its Correlation With Impaired Cognitive Functioning. J. Clin. Oncol. 2012, 30, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.-W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Iconomou, G.; Mega, V.; Koutras, A.; Iconomou, A.V.; Kalofonos, H.P. Prospective assessment of emotional distress, cognitive function, and quality of life in patients with cancer treated with chemotherapy. Cancer 2004, 101, 404–411. [Google Scholar] [CrossRef]
- Watkins, P.C.; Mathews, A.; Williamson, D.A.; Fuller, R.D. Mood-congruent memory in depression: Emotional priming or elaboration? J. Abnorm. Psychol. 1992, 101, 581–586. [Google Scholar] [CrossRef]
- Buckwalter, J.G.; Crooks, V.C.; Petitti, D.B. Cognitive performance of older women who have survived cancer. Int. J. Neurosci. 2005, 115, 1307–1314. [Google Scholar] [CrossRef]
- Lindner, O.C.; Phillips, B.; McGabe, M.C.; Mayers, A.; Wearden, A.; Varese, F.; Talmi, D. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology 2014, 28, 726–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falleti, M.; Sanfilippo, A.; Maruff, P.; Weigh, L.; Phillips, K.A. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain Cogn. 2005, 59, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Collins, B.; MacKenzie, J.; Tomiak, E.; Verma, S.; Bielajew, C. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: A prospective study. Psycho-Oncology 2008, 17, 122–130. [Google Scholar] [CrossRef]
- Schagen, S.B.; Muller, M.J.; Boogerd, W.; Mellenbergh, G.J.; Van Dam, F.S.A.M. Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J. Natl. Cancer Inst. 2006, 98, 1742–1745. [Google Scholar] [CrossRef]
- Heflin, L.H.; Meyerowitz, B.E.; Hall, P.; Lichtenstein, P.; Johansson, B.; Pedersen, N.L.; Gatz, M. Re: Cancer as a risk factor for dementia: A house built on shifting sand. J. Natl. Cancer Inst. 2005, 97, 1550–1551. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef]
- Van Dam, F.S.A.M.; Boogerd, W.; Schagen, S.B.; Muller, M.J.; Fortuyn, M.E.D.; Wall, E.V.; Rodenhuis, S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J. Natl. Cancer Inst. 1998, 90, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Downie, F.P.; Fan, H.G.M.; Houédé-Tchen, N.; Yi, Q.; Tannock, I.F. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: Evaluation with patient interview after formal assessment. Psychooncology 2006, 15, 921–930. [Google Scholar] [CrossRef]
- Sioka, C.; Kyritsis, A.P. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother. Pharmacol. 2009, 63, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, C.; Savard, J.; Ivers, H. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res. Treat. 2009, 116, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, L.J.; Hall, A.; Maguire, G.P.; Baum, M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ 1990, 301, 575–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shaughnessy, J.A. Chemotherapy-related cognitive dysfunction in breast cancer. Semin. Oncol. Nurs. 2003, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Parkin, A.J. Explorations in Cognitive Neuropsychology (Traducción Española: Exploraciones en Neuropsicología Cognitiva); Blackwell Publishers: London, UK; Editorial Médica Panamericana: Madrid, Spain, 1996. [Google Scholar]
- Arraras, J.I.; Martinez, M.; Manterota, A.; Lainez, N. El grupo de la calidad de vida de la EORTC. Psicooncologia 2004, 1, 87–98. [Google Scholar]
- Van Groen, T.; Kadish, I.; Popović, N.; Popović, M.; Caballero-Bleda, M.; Baño-Otálora, B.; Vivanco, P.; Ángeles-Rol, M.; Madrid, J.A. Age-related brain pathology in Octodon degu: Blood vessel, white matter and Alzheimer-like pathology. Neurobiol. Aging 2011, 32, 1651–1661. [Google Scholar] [CrossRef]
- Robins-Wahlin, T.-B.; Bäckman, L.; Wahlin, Å.; Winblad, B. Trail Making Test performance in a community-based sample of healthy very old adults: Effects of age on completion time, but not on accuracy. Arch. Gerontol. Geriatr. 1996, 22, 87–102. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 28, 643–662. [Google Scholar] [CrossRef]
- Booth, C.M.; Vardy, J.; Crawley, A.; Rourke, S.; Pond, G.; Wagner, L.; Tannock, I.F. Cognitive impairment associated with chemotherapy for breast cancer: An exploratory case-control study. J. Clin. Oncol. 2006, 24, 8501. [Google Scholar] [CrossRef]
- Boykoff, N.; Moieni, M.; Sumramanian, S.K. Confronting chemobrain: An in-depth look at survivors ’ reports of impact on work, social networks, and health care response. J. Cancer Surviv. 2009, 3, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Weiss, H.D.; Walker, M.D.; Wiernik, P.H. Neurotoxicity of commonly used antineoplastic agents. N. Engl. J. Med. 1974, 291, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Swayampakula, A.K.; Alkhouri, N.; Haut, M.W.; Abraham, J. Cognitive impairment with significant brain parenchymal volume loss following standard adjuvant chemotherapy in a patient with breast cancer. Clin. Adv. Hematol. Oncol. H&O 2007, 5, 985–987. [Google Scholar]
- Taillibert, S.; Voillery, D.; Bernard-Marty, C. Chemobrain: Is systemic chemotherapy neurotoxic? Curr. Opin. Oncol. 2007, 19, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Nandy, N.; Roth, A.J. Chemotherapy and cognitive deficits: Mechanisms, findings, and potential interventions. Palliat Support. Care 2007, 5, 273–280. [Google Scholar] [CrossRef]
- Wefel, J.S.; Lenzi, R.; Theriault, R.L.; Buzdar, A.U.; Cruickshank, S.; Meyers, C.A. “Chemobrain” in breast carcinoma? A prologue. Cancer 2004, 101, 468–475. [Google Scholar] [CrossRef]
- Vardy, J. Cognitive function in survivors of cancer. Am. Soc. Clin. Oncol. Educ. 2009, 1, 570–574. [Google Scholar] [CrossRef]
- Shilling, V.; Jenkins, V.; Morris, R.; Deutsch, D.; Bloomfield, D. The effects of adjuvant chemotherapy on cognition in women with breast cancer—Preliminary results of an observational longitudinal study. Breast 2005, 14, 142–150. [Google Scholar] [CrossRef]
- Yoshikawa, E.; Matsuoka, Y.; Inagaki, M.; Nakano, T.; Akechi, T.; Kobayakawa, M.; Fujimori, M.; Nakaya, N.; Akizuki, N.; Imoto, S.; et al. No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast Cancer Res. Treat. 2005, 92, 81–84. [Google Scholar] [CrossRef]
- A Meyers, C.; Abbruzzese, J.L. Cognitive functioning in cancer patients: Effect of previous treatment. Neurology 1992, 42, 434–436. [Google Scholar] [CrossRef]
- López-Santiago, S.; Cruzado, J.A.; Feliú, J. Chemobrain: Revisión de estudios que evalúan el deterioro cognitivo de supervivientes de cáncer tratados con quimioterapia. Psicooncología 2011, 8, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Kesler, S.R.; Kent, J.S.; O’Hara, R. Prefrontal Cortex and Executive Function Impairments in Primary Breast Cancer. Arch. Neurol. 2011, 68, 1447–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radziwillowicz, W.; Radziwillowicz, P. Subjective and objective assessment of memory functions in endogenous depression. Arch. Psychiatr. Psychother. 2000, 2, 33–41. [Google Scholar]
- Jalali, R.; Singh, S.; Budrukkar, A. Techniques of tumour bed boost irradiation in breast conserving therapy: Current evidence and suggested guidelines. Acta Oncol. 2007, 46, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Kowalski, T.L.; Chang, C.-H. Quality of life assessment in women with breast cancer: Benefits, acceptability and utilization. Health Qual. Life Outcomes 2007, 5, 24. [Google Scholar] [CrossRef] [Green Version]
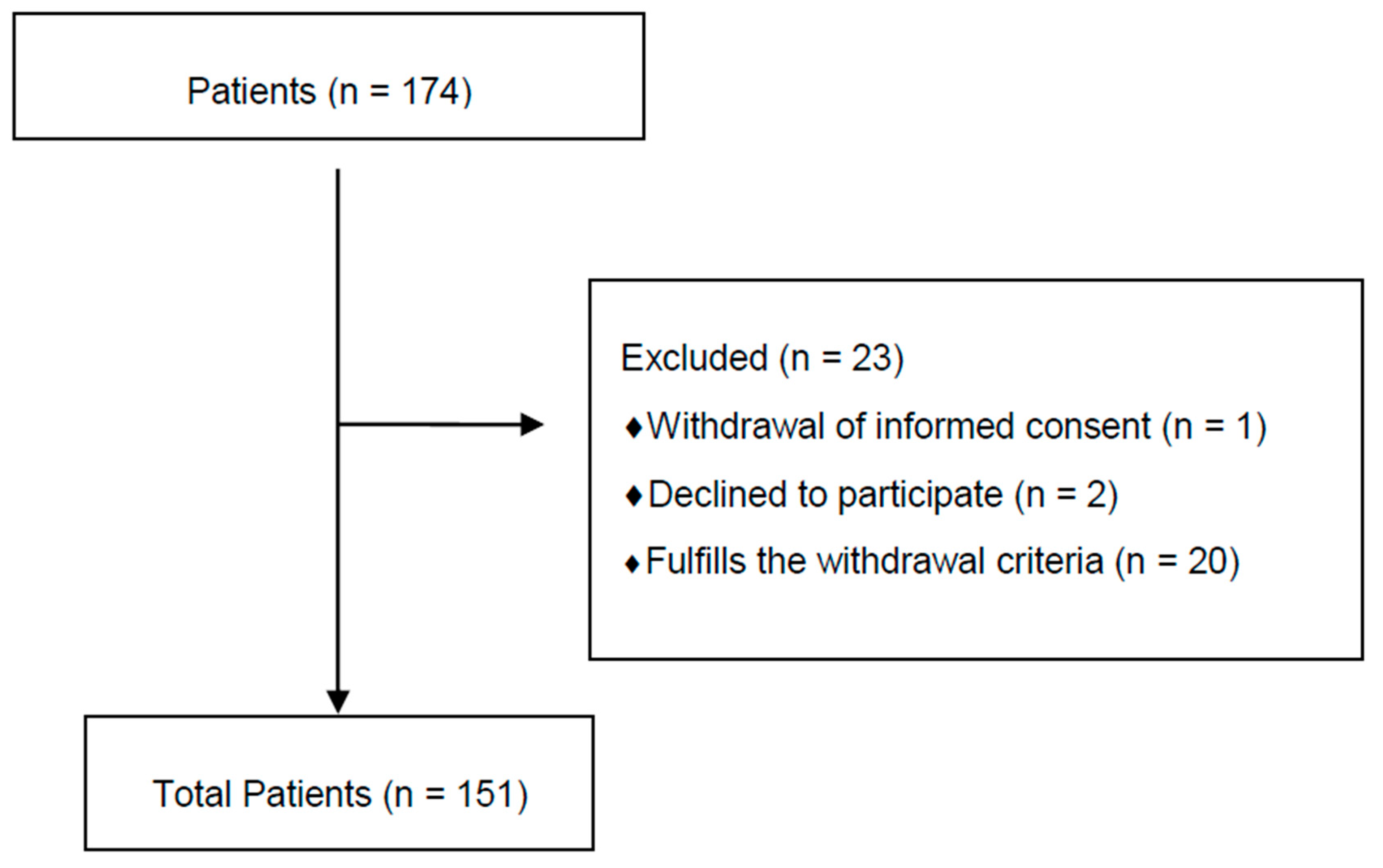
| Exclusion Criteria | Withdrawal Criteria |
|---|---|
| Having locoregional breast cancer stage IIIA or above | Nonresponse of all the items in the questionnaires |
| Previous chemotherapy | Psychopharmacological treatment |
| No current chemotherapy treatment | Not completing the study follow-up |
| No patient of Salamanca University Assistance Complex | Exitus |
| Being a minor | |
| Age older than 85 years | |
| Pregnancy | |
| Not signing the written informed consent | |
| Chronic insomnia | |
| Psychopathological diagnosis |
| Variables | M | SD | Minimum Score Obtained | Maximum Score Obtained |
|---|---|---|---|---|
| Vocabulary Before | 27.76 | 6.590 | 15 | 45 |
| Vocabulary During | 26.53 | 7.427 | 14 | 47 |
| Vocabulary After | 27.46 | 7.127 | 15 | 46 |
| Symbol search Before | 25.71 | 5.650 | 15 | 41 |
| Symbol search During | 23.41 | 5.110 | 14 | 37 |
| Symbol search After | 22.59 | 5.078 | 13 | 39 |
| Key Before | 44.15 | 10.708 | 28 | 81 |
| Key During | 38.32 | 9.524 | 25 | 74 |
| Key After | 35.84 | 9.156 | 23 | 77 |
| Before | 15.42 | 1.757 | 11 | 21 |
| L and N During | 13.58 | 1.741 | 9 | 19 |
| L and N After | 12.22 | 1.496 | 9 | 16 |
| TMT_A Before | 50.85 | 16.486 | 27 | 89 |
| TMT_A During | 53.95 | 16.845 | 29 | 91 |
| TMT_A After | 55.81 | 17.199 | 31 | 95 |
| TMT_B Before | 116.75 | 32.853 | 65 | 198 |
| TMT_B During | 121.42 | 33.086 | 66 | 69 |
| TMT_B After | 125.23 | 33.25 | 69 | 209 |
| Stroop word Before | 122.27 | 4.286 | 110 | 132 |
| Stroop word During | 117.77 | 3.921 | 108 | 127 |
| Stroop word After | 114.93 | 3.871 | 107 | 126 |
| Stroop color Before | 76.86 | 4.032 | 70 | 86 |
| Stroop color During | 70.62 | 4.415 | 60 | 81 |
| Stroop color After | 67.56 | 3.834 | 59 | 79 |
| Stroop color and word Before | 49.30 | 4.064 | 40 | 59 |
| Stroop color and word During | 43.75 | 3.912 | 33 | 53 |
| Stroop color and word After | 41.94 | 3.077 | 31 | 49 |
| Variables | HAD Emotional State: Anxiety and Depression | |||||
|---|---|---|---|---|---|---|
| 1. Normal n = 6 | 2. Borderline n = 62 | 3. Clinical Problem n = 83 | Scheffé | F | p | |
| M | M | M | ||||
| Search Symbol before | 26.00 | 27.24 | 24.54 | 2–3 * | 4.234 | p < 0.05 |
| L y N before | 17.00 | 15.73 | 15.08 | 1–3 * | 5.152 | p < 0.01 |
| TMT_A before | 38.83 | 44.24 | 56.66 | 1–3 * y 2–3 * | 13.725 | p < 0.001 |
| TMT_A during | 40.83 | 47.45 | 59.76 | 1–3 * y 2–3 * | 13.219 | p < 0.001 |
| TMT_A after | 42.67 | 49.03 | 61.82 | 1–3 * y 2–3 * | 13.585 | p < 0.001 |
| TMT_B before | 88.83 | 103.77 | 128.46 | 1–3 * y 2–3 * | 14.478 | p < 0.001 |
| TMT_B during | 93.33 | 107.97 | 133.49 | 1–3 * y 2–3 * | 15.247 | p < 0.001 |
| TMT_B after | 97.67 | 111.29 | 137.64 | 1–3 * y 2–3 * | 15.936 | p < 0.001 |
| Stroop word before | 123.83 | 123.39 | 121.33 | 2–3 * | 4.746 | p < 0.001 |
| Stroop Color and Word before | 50.83 | 50.65 | 48.18 | 2–3 * | 7.582 | p < 0.01 |
| Stroop Color and Word during | 44.17 | 45.10 | 42.72 | 2–3 * | 7.102 | p < 0.01 |
| Quality of Life. Scores of the EORTC QLQ-BR23 Scale | ||||||||
|---|---|---|---|---|---|---|---|---|
| BEFORE | DURING | AFTER | ||||||
| Low | Half | High | Low | Half | High | Low | Half | High |
| 0 (0%) | 19 (12.65%) | 1 (7%) | 15 (9.9%) | 5 (3.3%) | 0 (0%) | 15 (9.9%) | 5 (3.3%) | 0 (0%) |
| 4 (2.6%) | 49 (32.5%) | 12 (7.9%) | 9 (6%) | 55 (36.4%) | 1 (7%) | 1 (7%) | 62 (41.1%) | 2 (1.3) |
| 2 (1.3%) | 46 (30.5%) | 18 (11.9%) | 4 (2.6%) | 44 (29.1%) | 18 (11.9%) | 4 (2.6%) | 33 (21.9%) | 29 (19.2%) |
| 1 (0.7%) | 20 (13.2%) | 3 (2%) | 16 (10.6%) | 8 (5.3%) | 0 (0%) | 15 (9.9%) | 9 (6%) | 0 (0%) |
| 5 (3.3%) | 77 (51%) | 18 (11.9%) | 12 (7.9%) | 80 (53%) | 8 (5.3%) | 5 (3.3%) | 78 (51.7%) | 17 (11.3%) |
| 0 (0%) | 17 (11.3%) | 10 (6.6%) | 0 (0%) | 16 (10.6%) | 11 (7.3%) | 0 (0%) | 13 (8.6%) | 14 (9.3%) |
| 1 (7%) | 21 (13.9%) | 3 (2%) | 17 (11.3%) | 8 (5.3%) | 0 (0%) | 15 (9.9%) | 10 (6.6%) | 0 (0%) |
| 2 (1.3%) | 35 (23.2%) | 9 (6%) | 6 (4%) | 32 (21.2%) | 8 (5.3%) | 3 (2%) | 30 (19.9%) | 13 (8.6%) |
| 3 (2%) | 58 (38.4%) | 19 (12.6%) | 5 (3.3%) | 64 (42.4%) | 11 (7.3%) | 2 (1.3%) | 60 (39.7%) | 31 (20.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Martín, B.; Fernández Rodríguez, E.J.; Rihuete Galve, M.I.; Cruz Hernández, J.J. Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8896. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17238896
Rodríguez Martín B, Fernández Rodríguez EJ, Rihuete Galve MI, Cruz Hernández JJ. Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. International Journal of Environmental Research and Public Health. 2020; 17(23):8896. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17238896
Chicago/Turabian StyleRodríguez Martín, Blanca, Eduardo José Fernández Rodríguez, María Isabel Rihuete Galve, and Juan Jesús Cruz Hernández. 2020. "Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer" International Journal of Environmental Research and Public Health 17, no. 23: 8896. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17238896





