Effects of In-Hospital Physical Therapy on Activities of Daily Living in Patients with Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Subjects
2.4. Protocol for Physical Therapy
2.4.1. Resistance Training
2.4.2. Stretching
2.4.3. Aerobic Exercise
2.5. Diagnosis, Tumor Node Metastasis (TNM) Staging, and Treatment of HCC
2.6. Measurement of Psoas Muscle Index (PMI) and Skeletal Muscle Index (SMI)
2.7. Measurement of Muscle Strength
2.7.1. Maximum Muscle Strength Assessment of Knee Extension Strength
2.7.2. Grip Strength
2.7.3. 30-Second Chair–Stand Test (CS-30)
2.8. Measurement of Walking Speed
2.9. Measurement of FIM
2.10. Biochemical Tests
2.11. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes in Liver and Renal Function after Physical Therapy
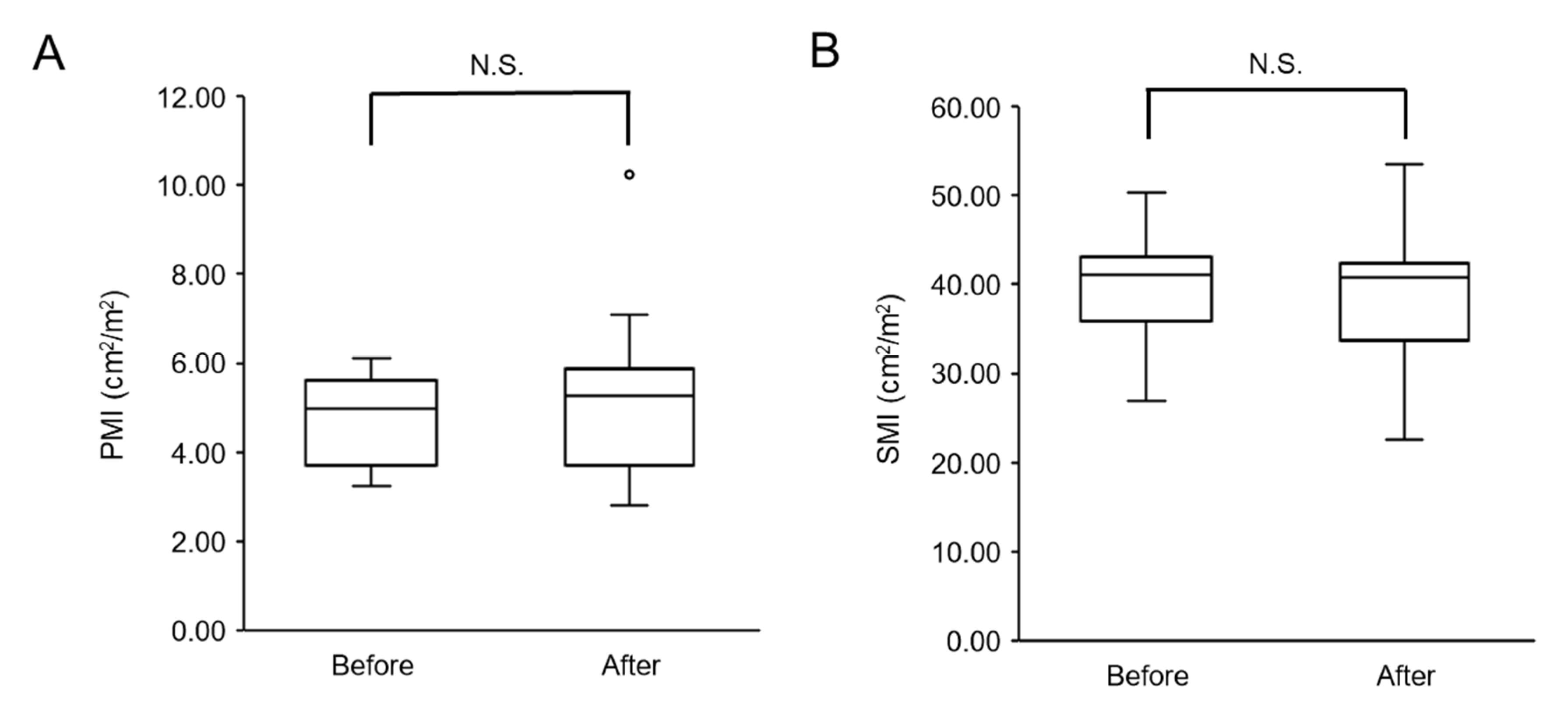
3.2.1. Changes in Skeletal Muscle Mass after Physical Therapy
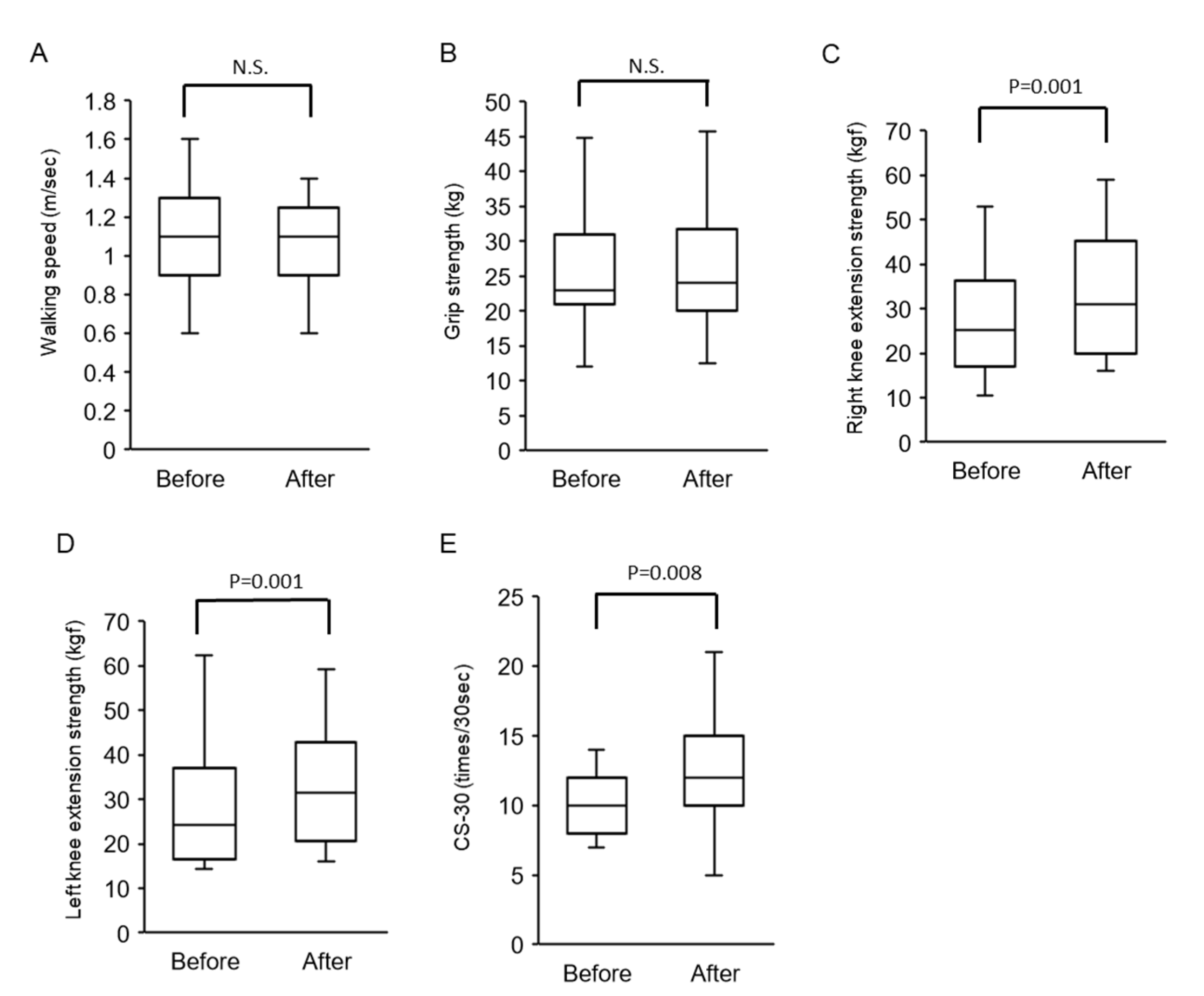
3.2.2. Changes in Walking Speed and Muscle Strength after Physical Therapy
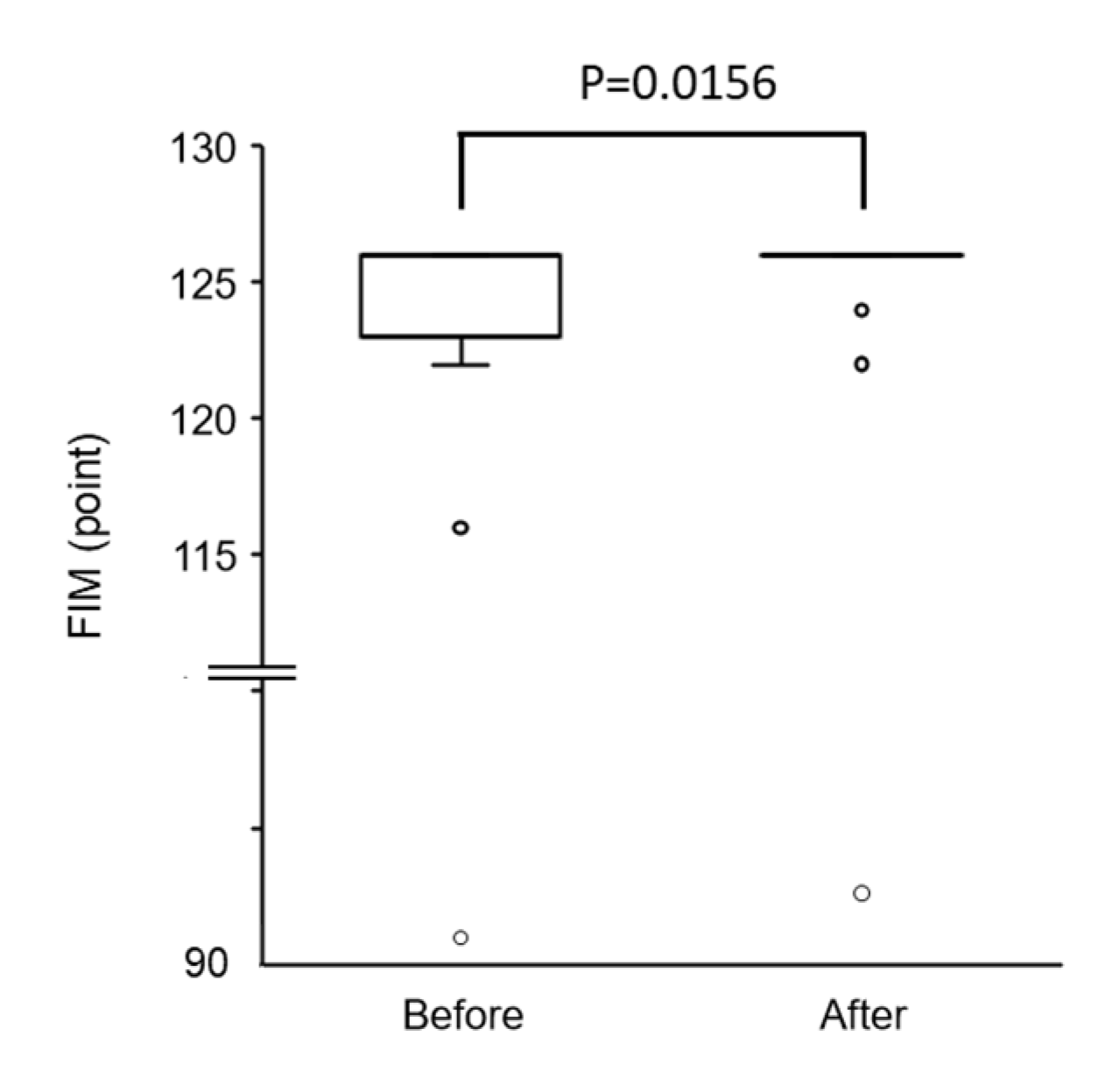
3.2.3. Changes in FIM Score and All Items of FIM after Physical Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kenis, C.; DeCoster, L.; Bastin, J.; Bode, H.; Van Puyvelde, K.; De Grève, J.; Conings, G.; Fagard, K.; Flamaing, J.; Milisen, K.; et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J. Geriatr. Oncol. 2017, 8, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Duchowny, K.A.; Clarke, P.; Peterson, M.D. Muscle Weakness and Physical Disability in Older Americans: Longitudinal Findings from the U.S. Health and Retirement Study. J. Nutr. Health Aging 2018, 22, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Van Ancum, J.M.; Scheerman, K.; Jonkman, N.H.; Smeenk, H.E.; Kruizinga, R.C.; Meskers, C.; Maier, A.B. Change in muscle strength and muscle mass in older hospitalized patients: A systematic review and meta-analysis. Exp. Gerontol. 2017, 92, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Kawaguchi, T.; Hashida, R.; Koya, S.; Bekki, M.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Nozoe, R.; Nakano, D.; et al. Profiles Associated with Sarcopenia in Hepatoma Patients Underwent Transcatheter Arterial Chemoembolization: A Data-Mining Analysis. JCSM Clin. Rep. 2018, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Franken, F.H. Bed rest, diet and working capability in liver disease (author’s transl). Leber. Magen Darm. 1977, 7, 300–305. [Google Scholar]
- Glenny, C.; Stolee, P. Comparing the Functional Independence Measure and the interRAI/MDS for use in the functional assessment of older adults: A review of the literature. BMC Geriatr. 2009, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Maritz, R.; Tennant, A.; Fellinghauer, C.; Stucki, G.; Prodinger, B. The Functional Independence Measure 18-item version can be reported as a unidimensional interval-scaled metric: Internal construct validity revisited. J. Rehabil. Med. 2019, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Bender, A.; Bauch, S.; Grill, E. Efficacy of a post-acute interval inpatient neurorehabilitation programme for severe brain injury. Brain Inj. 2013, 28, 44–50. [Google Scholar] [CrossRef]
- Hamilton, B.B.; A Laughlin, J.; Fiedler, R.C.; Granger, C.V. Interrater reliability of the 7-level functional independence measure (FIM). Scand. J. Rehabil. Med. 1994, 26, 115–119. [Google Scholar]
- McEwen, S.; Elmi, S.; Waldman, M.; Bishev, M. Inpatient oncology rehabilitation in Toronto: A descriptive 18-month retrospective record review. Support. Care Cancer 2011, 20, 1541–1547. [Google Scholar] [CrossRef]
- Wang, D.X.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachex- Sarcopenia Muscle 2019, 11, 3–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, J.A.; Tecci, M.G.; Lanni, M.A.; Fischer, J.P.; Fosnot, J.; Selber, J.C.; Wu, L.C.; Serletti, J.M. Function and Strength after Free Abdominally Based Breast Reconstruction: A 10-Year Follow-Up. Plast Reconstr. Surg. 2019, 143, 22e–31e. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.P.; Scialla, S.J.; Bednarz, L. Functional recovery in cancer rehabilitation. Arch. Phys. Med. Rehabil. 2000, 81, 623–627. [Google Scholar] [CrossRef]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Hirota, K.; Bekki, M.; Goto, E.; Yamada, M.; Sugimoto, M.; Hayashi, S.; Goshima, N.; et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J. Gastroenterol. Hepatol. 2018, 34, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Goto, E.; Matsuse, H.; Saito, H.; Hirota, K.; Taira, R.; Matsushita, Y.; Imanaga, M.; et al. Effects of In-hospital Exercise on Liver Function, Physical Ability, and Muscle Mass during Treatment of Hepatoma in Patients with Chronic Liver Disease. Hepatol. Res. 2016, 47, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashida, R.; Kawaguchi, T.; Koya, S.; Hirota, K.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Bekki, M.; Iwanaga, S.; Nakano, D.; et al. Impact of cancer rehabilitation on the prognosis of patients with hepatocellular carcinoma. Oncol. Lett. 2020, 19, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; E Davis, T.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Citro, V.; Milan, G.; Tripodi, F.S.; Gennari, A.; Sorrentino, P.; Gallotta, G.; Postiglione, A.; Tarantino, G. Mental status impairment in patients with West Haven grade zero hepatic encephalopathy: The role of HCV infection. J. Gastroenterol. 2007, 42, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports, M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar]
- Miyatake, N.; Saito, T.; Miyachi, M.; Tabata, I.; Numata, T. Evaluation of muscle strength and its relation to exercise habits in Japanese. Acta Med. Okayama 2009, 63, 151–155. [Google Scholar]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Arii, S.; Sata, M.; Sakamoto, M.; Shimada, M.; Kumada, T.; Shiina, S.; Yamashita, T.; Kokudo, N.; Tanaka, M.; Takayama, T.; et al. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol. Res. 2010, 40, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ohara, M.; Suda, G.; Kimura, M.; Maehara, O.; Shimazaki, T.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Kawagishi, N.; Nakai, M.; et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol. Res. 2020, 50, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Kawaguchi, T.; Koya, S.; Nagamatsu, A.; Tomita, M.; Hashida, R.; Nakano, D.; Niizeki, T.; Matsuse, H.; Shiba, N.; et al. Clinical utility of the Liver Frailty Index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol. Res. 2019, 50, 330–341. [Google Scholar] [CrossRef]
- Nagamatsu, A.; Kawaguchi, T.; Hirota, K.; Koya, S.; Tomita, M.; Hashida, R.; Kida, Y.; Narao, H.; Manako, Y.; Tanaka, D.; et al. Slow walking speed overlapped with low handgrip strength in chronic liver disease patients with hepatocellular carcinoma. Hepatol. Res. 2019, 49, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Murray, S.M.; Chapman, K.; Munro, B.; Tiedemann, A. Sit-to-Stand Performance Depends on Sensation, Speed, Balance, and Psychological Status in Addition to Strength in Older People. Journals Gerontol. Ser. A Boil. Sci. Med Sci. 2002, 57, M539–M543. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Murray, M.P. Gait as a total pattern of movement. Am. J. Phys. Med. 1967, 46, 290–333. [Google Scholar]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar]
- García-Pagàn, J.C.; Santos, C.; A Barberá, J.; Luca, A.; Roca, J.; Rodriguez-Roisin, R.; Bosch, J.; Rodes, J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 1996, 111, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Guevara, M.; Fernandez-Esparrach, G.; Bataller, R.; Gines, A.; Jiménez, W.; Ginès, P.; Rivera, F.; Arroyo, V.; Rodés, J. Impairment of renal function during moderate physical exercise in cirrhotic patients with ascites: Relationship with the activity of neurohormonal systems. Hepatology 1997, 25, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- Muderrisoglu, H.; Yilmaz, K.C.; Karacaglar, E.; Bal, U.; Aydinalp, A.; Moray, G.; Haberal, M. Preoperative Cardiac Risk Assessment in Patients Undergoing Liver Transplant Due to Hepatocellular Carcinoma: Should It Be Different? Exp. Clin. Transplant. 2017, 15, 65–68. [Google Scholar] [PubMed]
- Schott, N.; Johnen, B.; Holfelder, B. Effects of free weights and machine training on muscular strength in high-functioning older adults. Exp. Gerontol. 2019, 122, 15–24. [Google Scholar] [CrossRef]
- Mikami, Y.; Kouda, K.; Kawasaki, S.; Okada, K.-I.; Kawai, M.; Kitahata, Y.; Miyazawa, M.; Hirono, S.; Unno, M.; Tajima, F.; et al. Preoperative In-Hospital Rehabilitation Improves Physical Function in Patients with Pancreatic Cancer Scheduled for Surgery. Tohoku J. Exp. Med. 2020, 251, 279–285. [Google Scholar] [CrossRef]
- Saotome, T.; Klein, L.A.; Faux, S.G. Cancer rehabilitation: A barometer for survival? Support. Care Cancer 2015, 23, 3033–3041. [Google Scholar] [CrossRef]
- Ng, A.; Gupta, E.; Fontillas, R.C.; Bansal, S.; Williams, J.L.; Park, M.; Liu, D.; Fu, J.B.; Yadav, R.; Bruera, E. Patient-Reported Usefulness of Acute Cancer Rehabilitation. PM R 2017, 9, 1135–1143. [Google Scholar] [CrossRef]
- Jung, H.; Yamasaki, M. Association of lower extremity range of motion and muscle strength with physical performance of community-dwelling older women. J. Physiol. Anthr. 2016, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Matsufuji, S.; Shoji, T.; Yano, Y.; Tsujimoto, Y.; Kishimoto, H.; Tabata, T.; Emoto, M.; Inaba, M. Effect of Chair Stand Exercise on Activity of Daily Living: A Randomized Controlled Trial in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 17–24. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A. Chair-stand exercise improves post-stroke dysphagia. Geriatr. Gerontol. Int 2020. [Google Scholar] [CrossRef]
- Miyata, C.; Tsuji, T.; Tanuma, A.; Ishikawa, A.; Honaga, K.; Liu, M. Cancer Functional Assessment Set: A new tool for functional evaluation in cancer. Am. J. Phys. Med. Rehabil. 2014, 93, 656–664. [Google Scholar] [CrossRef] [PubMed]

| Type of Exercise | Physical Therapy | Exercise Intensity | Frequency |
|---|---|---|---|
| 1. Resistance training | Knee extension (Used Combit CB-1) | 60% of MVC | 2–3 days/week |
| Standing stepping | Bodyweight | 5 days/week | |
| Calf raise | Bodyweight | 5 days/week | |
| Half squat | Bodyweight | 5 days/week | |
| 2. Stretching | Lower limb stretching | Painless strength | 3 days/week |
| 3. Aerobic exercise | Walking | Borg scale 11–13 (Fairly light to somewhat hard) | Every day |
| Reference Value | Median (IQR) | Range (Min–Max) | |
|---|---|---|---|
| N | N/A | 19 | N/A |
| Age (years) | N/A | 78.0 (73.0–82.0) | 60–86 |
| Sex (female/male) | N/A | 6/13 | N/A |
| Body mass index (kg/m2) | 18.5–24.9 | 21.2 (20.7–26.5) | 16.8–31.5 |
| Performance status (0/1/2/3/4) | N/A | 73.7%/26.3%/0%/0%/0% (14/5/0/0/0) | N/A |
| FIM score (points) | N/A | 126 (123–126) | 92–126 |
| Walking assistance devise (yes/no) | N/A | 10.5%/89.5% (2/17) | N/A |
| HCC stage (I/II/III/IV) | N/A | 5.3%/84.1%/5.3%/5.3% (1/16/1/1) | N/A |
| HCV/HBV/others | N/A | 78.9%/15.8%/5.3% (15/3/1) | N/A |
| Hospitalization (days) | N/A | 10.0 (10.0–13.0) | 7.0–28.0 |
| Physical therapy (days) | N/A | 5.0 (5.0–6.0) | 4.0–15.0 |
| Implementation rate of knee extension (%) | N/A | 66.7 (51.8–77.5) | 33.0–100.0 |
| Implementation rate of standing stepping (%) | N/A | 66.7 (51.8–77.5) | 33.0–100.0 |
| Implementation rate of calf raise (%) | N/A | 66.7 (51.8–77.5) | 33.0–100.0 |
| Implementation rate of half squat (%) | N/A | 66.7 (51.8–77.5) | 33.0–100.0 |
| Implementation rate of stretching (%) | N/A | 66.7 (51.8–77.5) | 33.0–100.0 |
| Implementation rate of aerobic exercise (%) | N/A | 75.0 (56.0–90.0) | 0.0–100.0 |
| Hepatic encephalopathy (Yes/No) | N/A | 10.5%/89.5% (2/17) | N/A |
| Ascites (Yes/No) | N/A | 10.5%/89.5% (2/17) | N/A |
| Walking speed (m/sec) | N/A | 1.1 (0.9–1.3) | 0.6–1.6 |
| Six-minute walk test (m) | N/A | 300 (262–340) | 150.5–465.0 |
| Grip strength (women/men) (kg) | N/A | 17.1 (13.8–21.7) /28.0 (24.3–31.3) | 12.0–22.3 /21.0–44.8 |
| Grip strength (low/normal) | N/A | 42.1%/57.9% (8/11) | N/A |
| Right knee extension strength (kgf) | N/A | 25.2 (17.0–36.3) | 10.5–53.0 |
| Left knee extension strength (kgf) | N/A | 24.2 (16.5–36.9) | 14.3–62.3 |
| SMI (women/men) (cm2/m2) | N/A | 39.9 (35.8–42.7) /41.1 (39.3–42.2) | 26.9–50.2 /27.4–50.2 |
| SMI (muscle atrophy/non-muscle atrophy) | N/A | 26.3%/73.7% (5/14) | N/A |
| PMI (women/men) (cm2/m2) | N/A | 3.6 (3.4–4.6) /5.0 (4.9–5.6) | 3.25–6.11 /3.48–6.07 |
| Presence of sarcopenia (Yes/No) | N/A | 31.6%/68.4% (6/13) | N/A |
| CS-30 (times/30 s) | N/A | 10.0 (8.0–12.0) | 7–22 |
| Child-Pugh score (points) | N/A | 5 (5–6) | 5–12 |
| Child-Pugh class (A/B/C) | N/A | 78.9%/15.8%/5.3% (15/3/1) | N/A |
| Anti-diabetic medication (Yes/No) | N/A | 26.3%/73.7% (5/14) | N/A |
| BCAA supplementation (Yes/No) | N/A | 31.6%/68.4% 6/13 | N/A |
| Biochemical examinations | |||
| Platelet count (× 104/μL) | 13.3–36.9 | 11.0 (7.4–17.6) | 4.8–26.7 |
| AST (U/L) | 10–40 | 33.0 (29.0–52.0) | 16.0–79.0 |
| ALT (U/L) | 5–40 | 26.0 (18.0–43.0) | 13.0–70.0 |
| Lactate dehydrogenase (U/L) | 115–245 | 213.0 (186.0–255.0) | 166.0–355.0 |
| ALP (U/L) | 115–359 | 377.0 (276.0–522.0) | 192.0–870.0 |
| GGT (U/L) | 0–30 | 37.0 (23.0–69.0) | 15.0–187.0 |
| Albumin (g/dL) | 4.0–5.0 | 3.7 (3.0–4.1) | 2.6–4.6 |
| Prothrombin activity (%) | 70–130 | 79.2 (67.1–86.8) | 55.0–102.0 |
| Total bilirubin (mg/dL) | 0.30–1.20 | 0.8 (0.6–0.9) | 0.3–3.1 |
| AFP (ng/mL) | 0–10 | 13.3 (5.8–40.2) | 3.5–1216.7 |
| des-γ-carboxy prothrombin (mAU/mL) | 0–39 | 107 (21.0–352.0) | 15–618 |
| BUN (mg/dL) | 8.0–22.0 | 15.5 (13.5–17.8) | 6.4–28.1 |
| Creatinine (mg/dL) | 0.61–1.04 | 0.8 (0.6–1.0) | 0.5–1.36 |
| eGFR (mL/min/1.73 m2) | >90.0 | 65.1 (55.0–82.9) | 39.1–90.0 |
| Sodium (mmol/L) | 138–145 | 139 (138.0–141.0) | 134–142 |
| Potassium (mmol/L) | 3.6–4.8 | 4.0 (3.7–4.2) | 2.9–5.2 |
| HbA1c (%) | 4.3–5.8 | 5.6 (5.4–6.8) | 4.5–7.8 |
| Ammonia (μg/dL) | 13–86 | 79.0 (52.7–134.2) | 40.0–156.0 |
| Before | After | ||||
|---|---|---|---|---|---|
| Median (IQR) | Range (Min–Max) | Median (IQR) | Range (Min–Max) | p | |
| Child-Pugh score (point) | 5 (5–6) | 5–12 | 6 (6–7) | 5–10 | 0.112 |
| Child-Pugh class (A/B/C) | 78.9%/15.8%/5.3% (15/3/1) | N/A | 63.1%/31.6%/5.3% (12/6/1) | N/A | 0.180 |
| AST (U/L) | 33.0 (29.0–52.0) | 16.0–79.0 | 37.0 (28.0–49.0) | 21.0–114.0 | 0.7210 |
| ALT (U/L) | 26.0 (18.0–43.0) | 13.0–70.0 | 37.0 (23.0–56.0) | 15.0–99.0 | 0.2579 |
| Lactate dehydrogenase (U/L) | 213.0 (186.0–255.0) | 166.0–355.0 | 198.0 (171.0–246.0) | 148.0–392.0 | 0.3225 |
| ALP (U/L) | 377.0 (276.0–522.0) | 192.0–870.0 | 382.0 (305.0–479.0) | 158.0–511.0 | 0.1703 |
| GGT (U/L) | 37.0 (23.0–69.0) | 15.0–187.0 | 53.0 (29.0–82.0) | 14.0–203.0 | 0.4247 |
| eGFR (mL/min/1.73 m2) | 65.1 (52.8–84.3) | 39.1–90.0 | 65.8 (57.9–84.6) | 34.0–99.9 | 0.2293 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narao, H.; Hirota, K.; Koya, S.; Tomita, M.; Manako, Y.; Ogawa, S.; Nakao, N.; Tsutsumi, T.; Nakano, D.; Hashida, R.; et al. Effects of In-Hospital Physical Therapy on Activities of Daily Living in Patients with Hepatocellular Carcinoma. Int. J. Environ. Res. Public Health 2020, 17, 9098. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17239098
Narao H, Hirota K, Koya S, Tomita M, Manako Y, Ogawa S, Nakao N, Tsutsumi T, Nakano D, Hashida R, et al. Effects of In-Hospital Physical Therapy on Activities of Daily Living in Patients with Hepatocellular Carcinoma. International Journal of Environmental Research and Public Health. 2020; 17(23):9098. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17239098
Chicago/Turabian StyleNarao, Hayato, Keisuke Hirota, Shunji Koya, Manabu Tomita, Yuta Manako, Satosi Ogawa, Naomi Nakao, Tsubasa Tsutsumi, Dan Nakano, Ryuki Hashida, and et al. 2020. "Effects of In-Hospital Physical Therapy on Activities of Daily Living in Patients with Hepatocellular Carcinoma" International Journal of Environmental Research and Public Health 17, no. 23: 9098. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17239098





