Metal Fractionation in Surface Sediments of the Brahmaputra River and Implications for Their Mobilization
Abstract
:1. Introduction
2. Study Area: Site Description and Geology
3. Materials and Methods
3.1. Sampling
3.2. Sequential Extraction
3.3. Analysis
3.4. Quality Assessment and Quality Control
3.5. Risk Assessment Code (RAC)
4. Results
4.1. General Characteristics of Water and Sediment
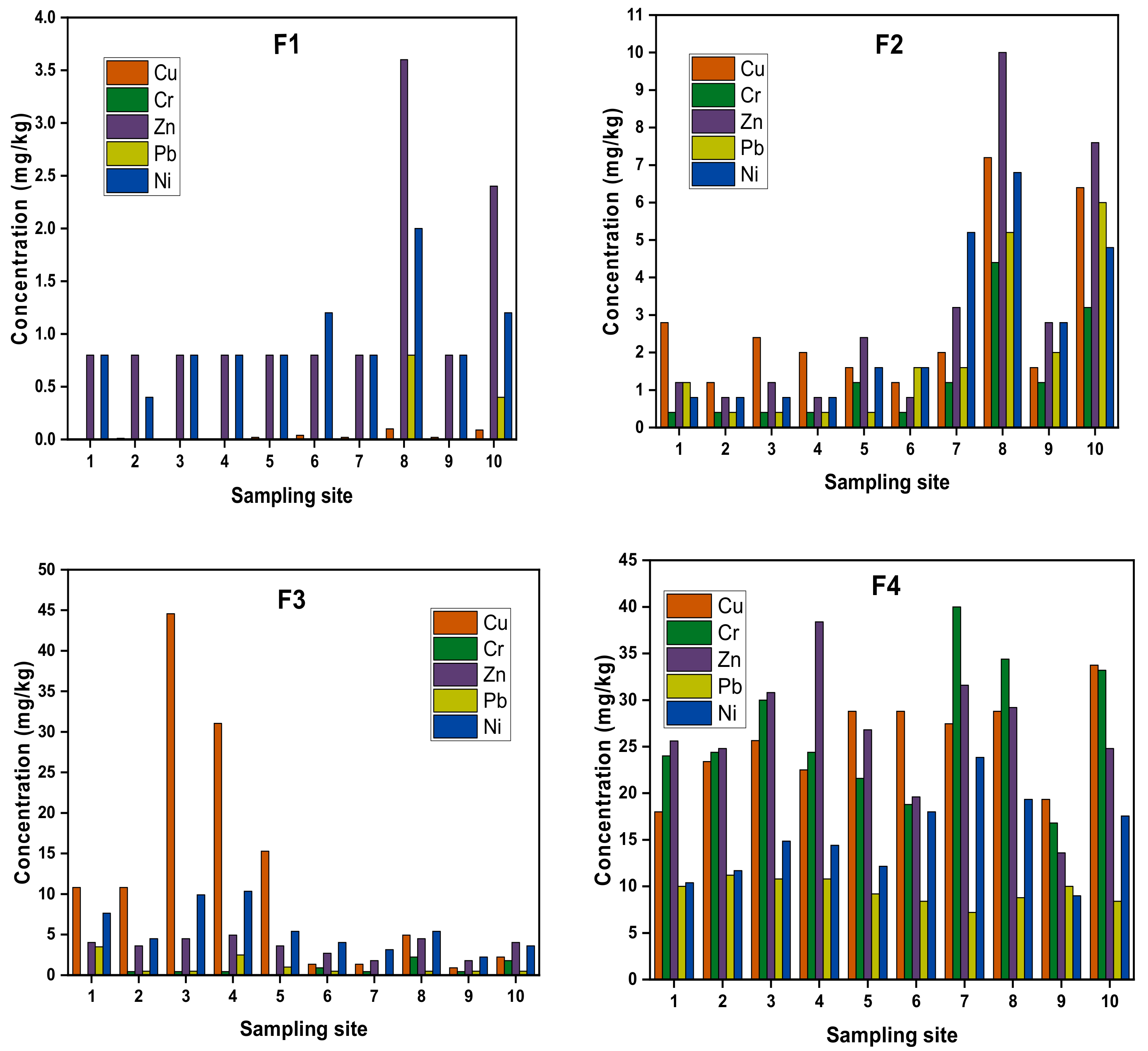
4.2. Chemical Fractionation
5. Discussion
5.1. The Interrelationship between the Overlying Water and Metal Species in the Sediment
5.1.1. Principal Component Analysis
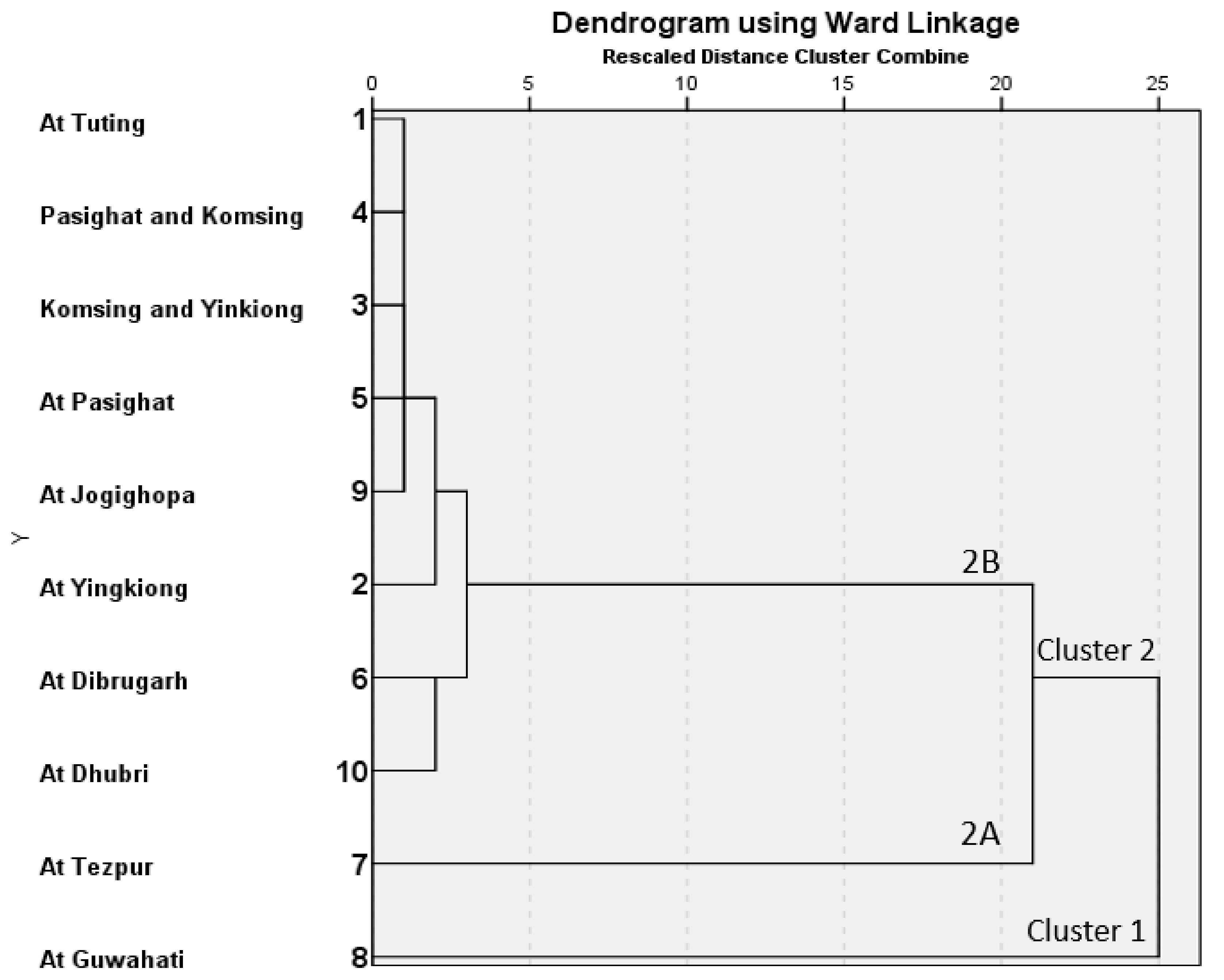
5.1.2. Cluster Analysis
5.2. Risk Assessment Code (RAC) and Environmental Implications for the Metal Fractionation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frémion, F.; Bordas, F.; Mourier, B.; Lenain, J.F.; Kestens, T.; Courtin-Nomade, A. Influence of dams on sediment continuity: A study case of a natural metallic contamination. Sci. Total Environ. 2016, 547, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ding, S.; Chen, X.; Sun, Q.; Fan, X.; Lin, J.; Ren, M.; Yang, L.; Zhang, C. Mechanisms driving phosphorus release during algal blooms based on hourly changes in iron and phosphorus concentrations in sediments. Water Res. 2018, 133, 153–164. [Google Scholar] [CrossRef]
- Artifon, V.; Zanardi-Lamardo, E.; Fillmann, G. Aquatic organic matter: Classification and interaction with organic microcontaminants. Sci. Total Environ. 2019, 649, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, V.; Pagnanelli, F.; Bornoroni, L.; Toro, L.; Abbruzzese, C. Toxic elements at a disused mine district: Particle size distribution and total concentration in stream sediments and mine tailings. J. Hazard. Mater. 2007, 148, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Tian, Y.; Peng, S.; Wang, M. Effect of dissolved oxygen and nutrient levels on heavy metal contents and fractions in river surface sediments. Sci. Total Environ. 2019, 648, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2008, 407, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Nemati, K.; Kartini, N.; Bakar, A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Borah, R.; Taki, K.; Gogoi, A.; Das, P.; Kumar, M. Contemporary distribution and impending mobility of arsenic, copper and zinc in a tropical (Brahmaputra) river bed sediments, Assam, India. Ecotoxicol. Environ. Saf. 2018, 161, 769–776. [Google Scholar] [CrossRef]
- Vosoogh, A.; Saeedi, M.; Lak, R. Metal fractionation and pollution risk assessment of different sediment sizes in three major southwestern rivers of Caspian Sea. Environ. Earth Sci. 2017, 76, 53. [Google Scholar] [CrossRef]
- Vosoogh, A.; Saeedi, M.; Lak, R. Heavy metals relationship with water and size-fractionated sediments in rivers using canonical correlation analysis (CCA) case study, rivers of south western Caspian Sea. Environ. Monit. Assess. 2016, 188, 603. [Google Scholar] [CrossRef]
- Davutluoglu, O.I.; Seckin, G.; Ersu, C.B.; Yilmaz, T.; Sari, B. Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J. Environ. Manag. 2011, 92, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Taki, K.; Kumar, M. Seasonal dynamics of metal phase distributions in the perennial tropical (Brahmaputra) river: Environmental fate and transport perspective. Environ. Res. 2020, 183, 109265. [Google Scholar] [CrossRef]
- Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Liu, Q.; Jia, Z.; Li, S.; Hu, J. Assessment of heavy metal pollution, distribution and quantitative source apportionment in surface sediments along a partially mixed estuary (Modaomen, China). Chemosphere 2019, 225, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef]
- Pallavi, D.; Kumar, M.; Sarma, K.P. Speciation of Heavy Metals in Surface Sediment of the Brahmaputra River, Assam, India. J. Environ. Res. Dev. 2015, 9, 944–952. [Google Scholar] [CrossRef]
- Berner, E.K.; Berner, R.A. Global Environment: Water, Air and Geochemical Cycles; Prentice-Hall: Upper Saddle River, NJ, USA, 1996; p. 376. [Google Scholar]
- Singh, S.K.; Sarin, M.M.; France-Lanord, C. Chemical erosion in the eastern Himalaya: Major ion composition of the Brahmaputra and δ13C of dissolved inorganic carbon. Geochim. Cosmochim. Acta 2005, 69, 3573–3588. [Google Scholar] [CrossRef]
- PTI. Causes behind Brahmaputra Turning Black Could Be Natural: Union Minister. 2017. Available online: https://economictimes.indiatimes.com/news/politics-and-nation/causes-behind-brahmaputra-turning-black-could-be-natural-union-minister/articleshow/61911643.cms (accessed on 4 December 2017).
- PTI. Brahmaputra River In Assam Has Changed Its Colour and Has Turned Muddy. 2017. Available online: https://swachhindia.ndtv.com/brahmaputra-river-assam-changed-colour-turned-muddy-15567/ (accessed on 7 December 2017).
- Verma, S.; Mukherjee, A.; Mahanta, C.; Choudhury, R.; Mitra, K. Influence of geology on groundwater–sediment interactions in arsenic enriched tectono-morphic aquifers of the Himalayan Brahmaputra river basin. J. Hydrol. 2016, 540, 176–195. [Google Scholar] [CrossRef]
- Garzanti, E.; Vezzoli, G.; Ando, S.; France-lanord, C.; Singh, S.K.; Foster, G. Sand petrology and focused erosion in collision orogens: The Brahmaputra case. Earth Planet. Sci. Lett. 2004, 220, 157–174. [Google Scholar] [CrossRef]
- Goodbred, S.L.; Paolo, P.M.; Ullah, M.S.; Pate, R.D.; Khan, S.R.; Kuehl, S.A.; Singh, S.K.; Rahaman, W. Piecing together the Ganges-Brahmaputra-Meghna river delta: Use of sediment provenance to reconstruct the history and interaction of multiple fluvial systems during Holocene delta evolution. Bull. Geol. Soc. Am. 2014, 126, 1495–1510. [Google Scholar] [CrossRef]
- Singh, S.K.; France-Lanord, C. Tracing the distribution of erosion in the Brahmaputra watershed from isotopic compositions of stream sediments. Earth Planet. Sci. Lett. 2002, 202, 645–662. [Google Scholar] [CrossRef]
- Burg, J.-P.; Oberli, F.; Maurin, J.-C.; Davy, P.; Burg, J.-P.; Diao, Z.; Meier, M.; Seward, D.; Nievergelt, P. The Namche Barwa syntaxis: Evidence for exhumation related to compressional crustal folding. J. Asian Earth Sci. 1998, 16, 239–252. [Google Scholar] [CrossRef]
- Verma, S.; Mukherjee, A.; Mahanta, C.; Choudhury, R. Applied Geochemistry Arsenic fate in the Brahmaputra river basin aquifers: Controls of geogenic processes, provenance and water-rock interactions. Appl. Geochem. 2019, 107, 171–186. [Google Scholar] [CrossRef]
- Gansser, A. Geology of the Himalayas; Wiley Interscience: New York, NY, USA, 1964. [Google Scholar]
- Dutta, S.K.; Gill, G.K.S.; Srinivasan, J. Geology of the Subansiri and Kamala Valleys; Geological Survey of India: Kolkata, India, 1983; pp. 8–14. [Google Scholar]
- Tipper, E.T.; Bickle, M.J.; Galy, A.; West, A.J.; Pomiès, C.; Chapman, H.J. The short term climatic sensitivity of carbonate and silicate weathering fluxes: Insight from seasonal variations in river chemistry. Geochim. Cosmochim. Acta 2006, 70, 2737–2754. [Google Scholar] [CrossRef]
- Zimmerman, A.J.; Weindorf, D.C. Heavy Metal and Trace Metal Analysis in Soil by Sequential Extraction: A Review of Procedures. Int. J. Anal. Chem. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Gui, S.; Huang, H.; Zhang, H.; Wang, C.; Guo, W. Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere 2017, 175, 473–481. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Liu, L.Z.; Shi, W.L.; Meng, X.Z. Comprehensive risk assessment of heavy metals in lake sediment from public parks in Shanghai. Ecotoxicol. Environ. Saf. 2014, 102, 129–135. [Google Scholar] [CrossRef]
- Ioka, S.; Muraoka, H.; Suzuki, Y. Redox potential of shallow groundwater by 1-month continuous in situ potentiometric measurements. Appl. Water Sci. 2017, 7, 3095–3101. [Google Scholar] [CrossRef] [Green Version]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters Surface and Ground Water Environments, 3rd ed.; Printice hall: Upper Saddle river, NJ, USA, 1997; pp. 154–156. [Google Scholar]
- Jain, C.K. Metal fractionation study on bed sediments of River Yamuna, India. Water Res. 2004, 38, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.A.; Jolley, D.F.; Simpson, S.L. Effect of overlying water pH, dissolved oxygen, salinity and sediment disturbances on metal release and sequestration from metal contaminated marine sediments. Chemosphere 2007, 69, 1428–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.L.; Apte, S.C.; Batley, G.E. Effect of short-term resuspension events on trace metal speciation in polluted anoxic sediments. Environ. Sci. Technol. 1998, 32, 620–625. [Google Scholar] [CrossRef]
- Vidal-Durà, A.; Burke, I.T.; Stewart, D.I.; Mortimer, R.J.G. Reoxidation of estuarine sediments during simulated resuspension events: Effects on nutrient and trace metal mobilisation. Estuar. Coast. Shelf Sci. 2018, 207, 40–55. [Google Scholar] [CrossRef]
- Deka, R.L.; Mahanta, C.; Pathak, H.; Nath, K.K.; Das, S. Trends and fluctuations of rainfall regime in the Brahmaputra and Barak basins of Assam, India. Theor. Appl. Climatol. 2013, 114, 61–71. [Google Scholar] [CrossRef]
- Kalnejais, L.H.; Martin, W.R.; Signell, R.P.; Bothner, M.H. Role of sediment resuspension in the remobilization of particulate-phase metals from coastal sediments. Environ. Sci. Technol. 2007, 41, 2282–2288. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, Z.; Tao, F.; Liu, B.; Tao, Z.; Gao, S. Characteristics of carbonate, evaporite and silicate weathering in Huanghe River basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Distribution of metals in water and suspended particulate matter during the resuspension processes in Taihu Lake sediment, China. Quat. Int. 2013, 286, 94–102. [Google Scholar] [CrossRef]
- Louis, Y.; Garnier, C.; Lenoble, V.; Mounier, S.; Cukrov, N.; Omanović, D.; Pižeta, I. Kinetic and equilibrium studies of copper-dissolved organic matter complexation in water column of the stratified Krka River estuary (Croatia). Mar. Chem. 2009, 114, 110–119. [Google Scholar] [CrossRef]
- Charriau, A.; Lesven, L.; Gao, Y.; Leermakers, M.; Baeyens, W.; Ouddane, B.; Billon, G. Trace metal behaviour in riverine sediments: Role of organic matter and sulfides. Appl. Geochem. 2011, 26, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Ciazela, J.; Siepak, M.; Wojtowicz, P. Tracking heavy metal contamination in a complex river-oxbow lake system: Middle Odra Valley, Germany/Poland. Sci. Total Environ. 2018, 616–617, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Luther, G.W.; Church, T.M. An overview of the environmental chemistry of sulphur in wetland systems. In Sulphur Cycling on the Continents; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Paul, A.; Deka, J.; Gujre, N.; Rangan, L.; Mitra, S. Does nature of livelihood regulate the urban community’s vulnerability to climate change? Guwahati city, a case study from North East India. J. Environ. Manag. 2019, 251, 109591. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, T.; Das, S. Urban flood mitigation planning for Guwahati: A case of Bharalu basin. J. Environ. Manag. 2018, 206, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
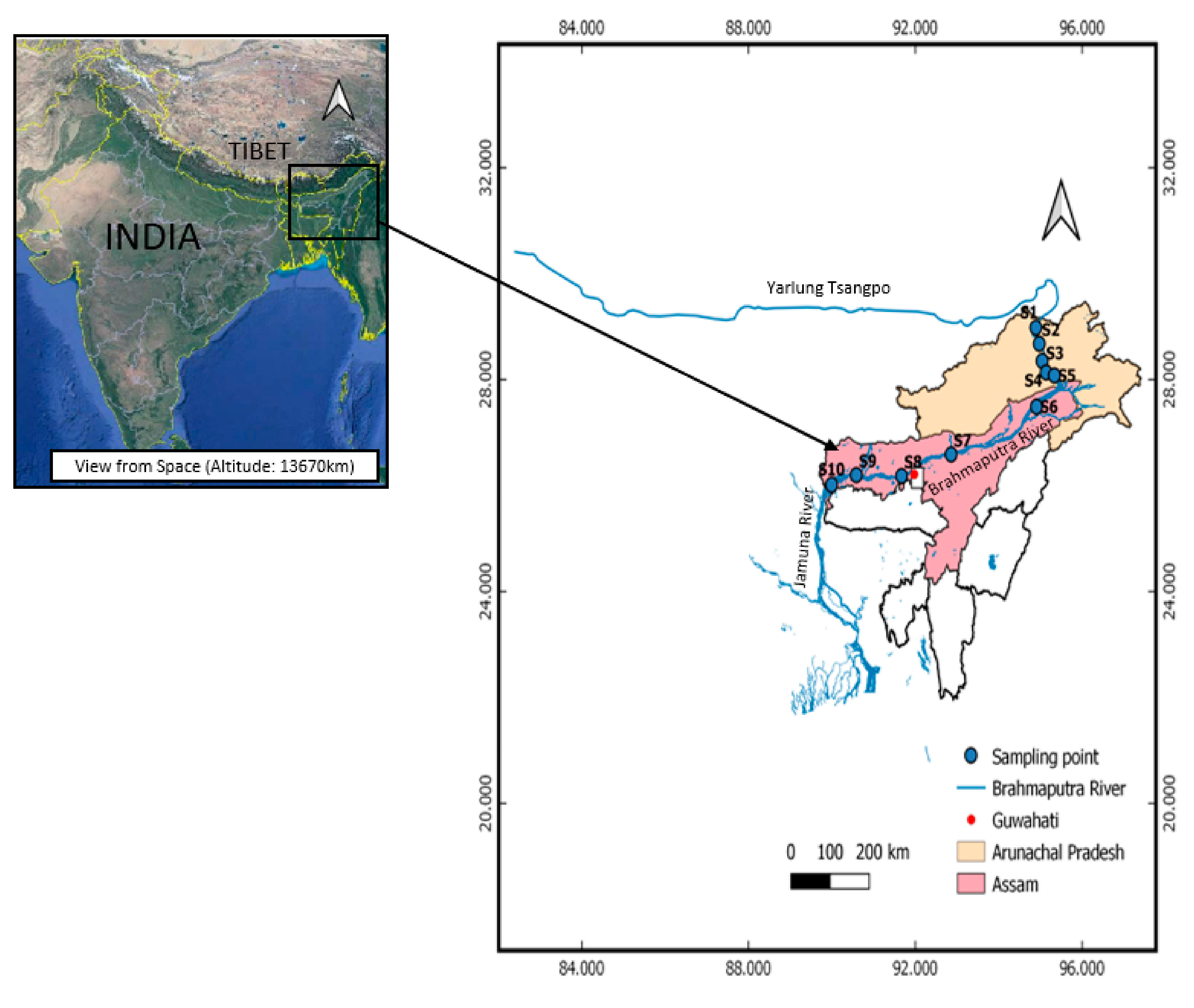

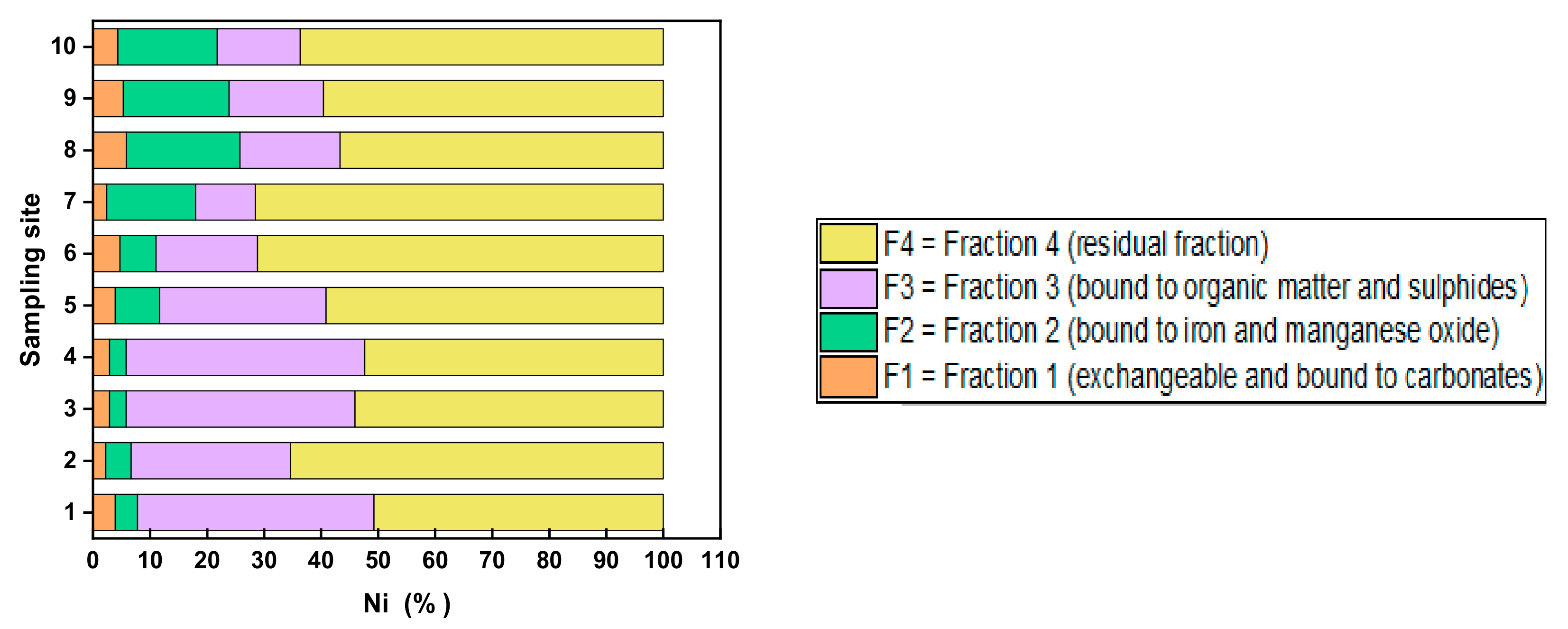
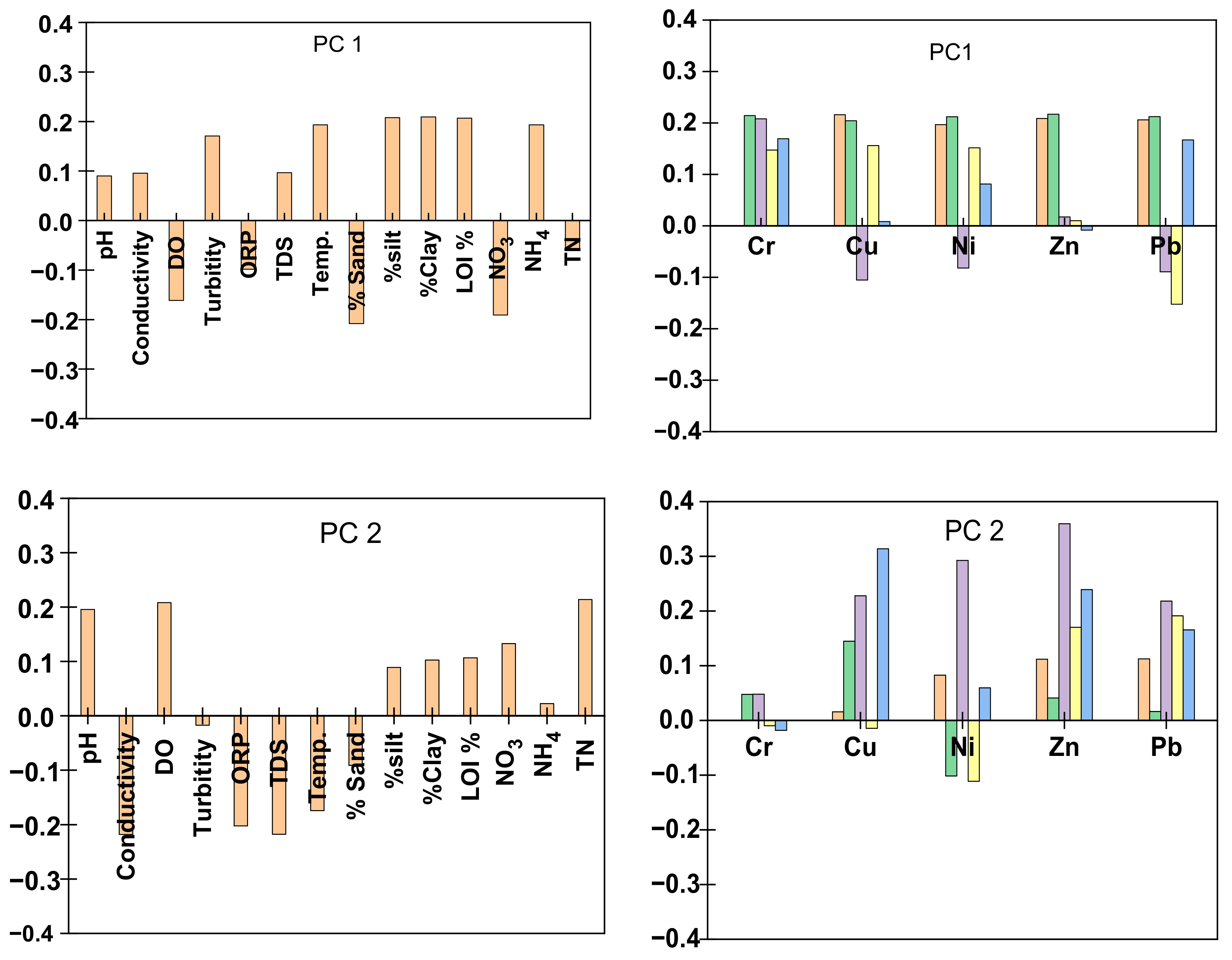
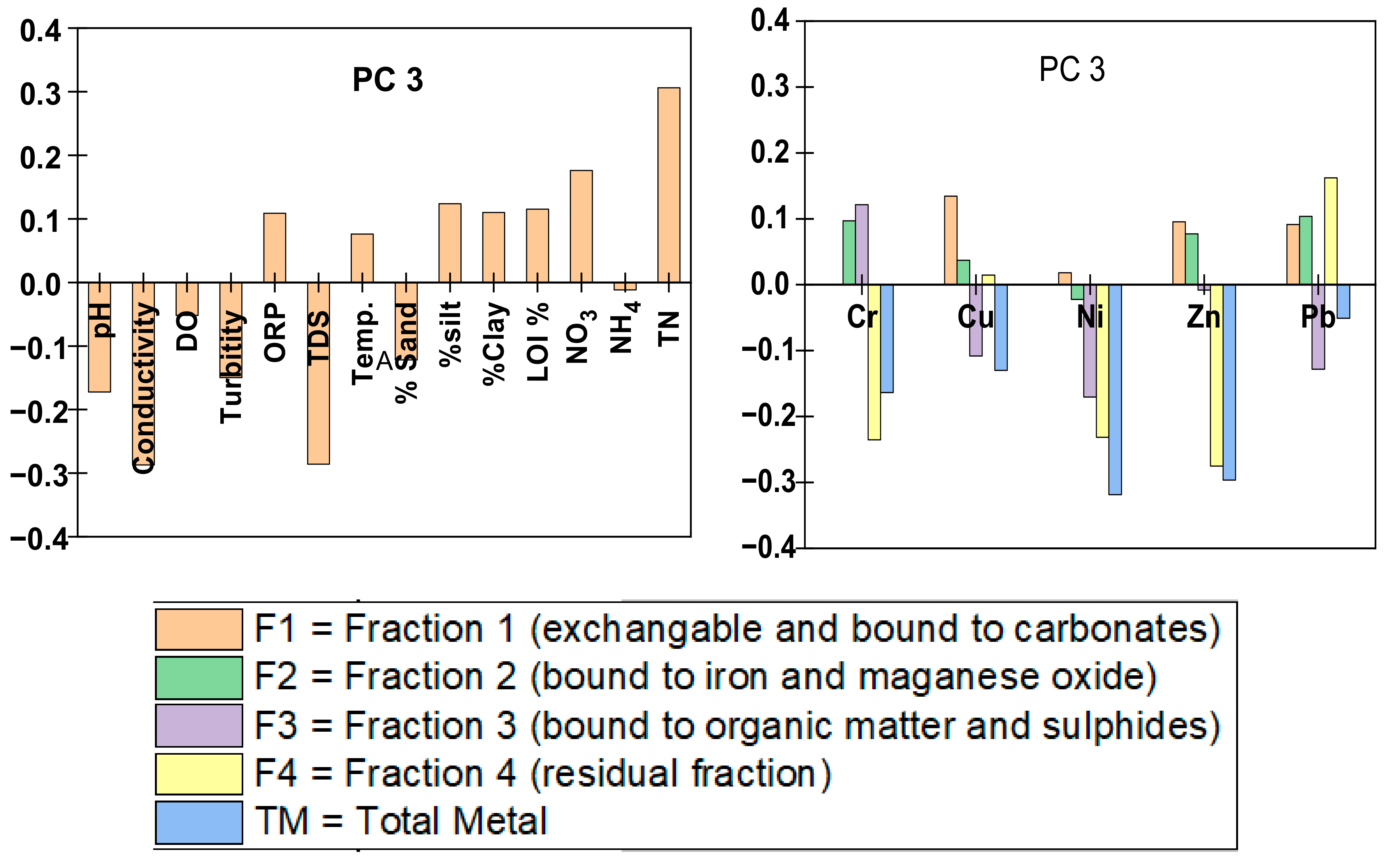
| Sediment No. | Date | Latitude | Longitude | Elevation (m) | pH | Conduc- Tivity (uS) | DO (mg/L) | Turbidity (NTU) | Measured Eh (mV) | TDS (ppm) | Temp. (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 13/11/2018 | 28.98097 | 94.89255 | 414 | 7.98 | 383.8 | 11.29 | 207 | 135.78 | 90.84 | 12.6 |
| S2 | 12/11/2018 | 28.677 | 94.97366 | 287 | 7.52 | 278.5 | 10.52 | 72.9 | 146.81 | 65.60 | 15.7 |
| S3 | 11/11/2018 | 28.35093 | 95.04578 | 215 | 7.93 | 353.2 | 12.00 | 258 | 135.76 | 82.60 | 14.2 |
| S4 | 11/11/2018 | 28.13514 | 95.14008 | 168 | 7.78 | 349.8 | 11.54 | 179 | 143.33 | 82.80 | 14.1 |
| S5 | 10/11/2018 | 28.07835 | 95.33553 | 132 | 7.97 | 312.6 | 11.50 | 207 | 135.70 | 73.93 | 15.0 |
| S6 | 9/11/2018 | 27.48809 | 94.90676 | 83.0 | 8.08 | 346.5 | 8.490 | 361 | 120.40 | 82.10 | 22.0 |
| S7 | 20/11/2018 | 26.58656 | 92.85958 | 57.0 | 7.73 | 897.1 | 9.050 | 557 | 141.31 | 211.0 | 22.7 |
| S8 | 21/11/2018 | 26.18033 | 91.67194 | 23.0 | 8.04 | 439.1 | 8.820 | 875 | 120.19 | 104.1 | 24.3 |
| S9 | 22/11/2018 | 26.1983 | 90.58509 | 27.0 | 7.39 | 381.7 | 10.04 | 134 | 160.72 | 90.34 | 20.4 |
| S10 | 22/11/2018 | 26.01339 | 89.99179 | 15.0 | 7.92 | 473.9 | 9.260 | 194 | 125.84 | 112.4 | 25.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsering, T.; Sillanpää, M.; Reinikainen, S.-P.; Abdel Wahed, M.S.M. Metal Fractionation in Surface Sediments of the Brahmaputra River and Implications for Their Mobilization. Int. J. Environ. Res. Public Health 2020, 17, 9214. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17249214
Tsering T, Sillanpää M, Reinikainen S-P, Abdel Wahed MSM. Metal Fractionation in Surface Sediments of the Brahmaputra River and Implications for Their Mobilization. International Journal of Environmental Research and Public Health. 2020; 17(24):9214. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17249214
Chicago/Turabian StyleTsering, Tenzin, Mika Sillanpää, Satu-Pia Reinikainen, and Mahmoud S. M. Abdel Wahed. 2020. "Metal Fractionation in Surface Sediments of the Brahmaputra River and Implications for Their Mobilization" International Journal of Environmental Research and Public Health 17, no. 24: 9214. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17249214






