Therapist Driven Rehabilitation Protocol for Patients with Chronic Heart and Lung Diseases: A Real-Life Study
Abstract
:1. Introduction
2. Materials and Methods
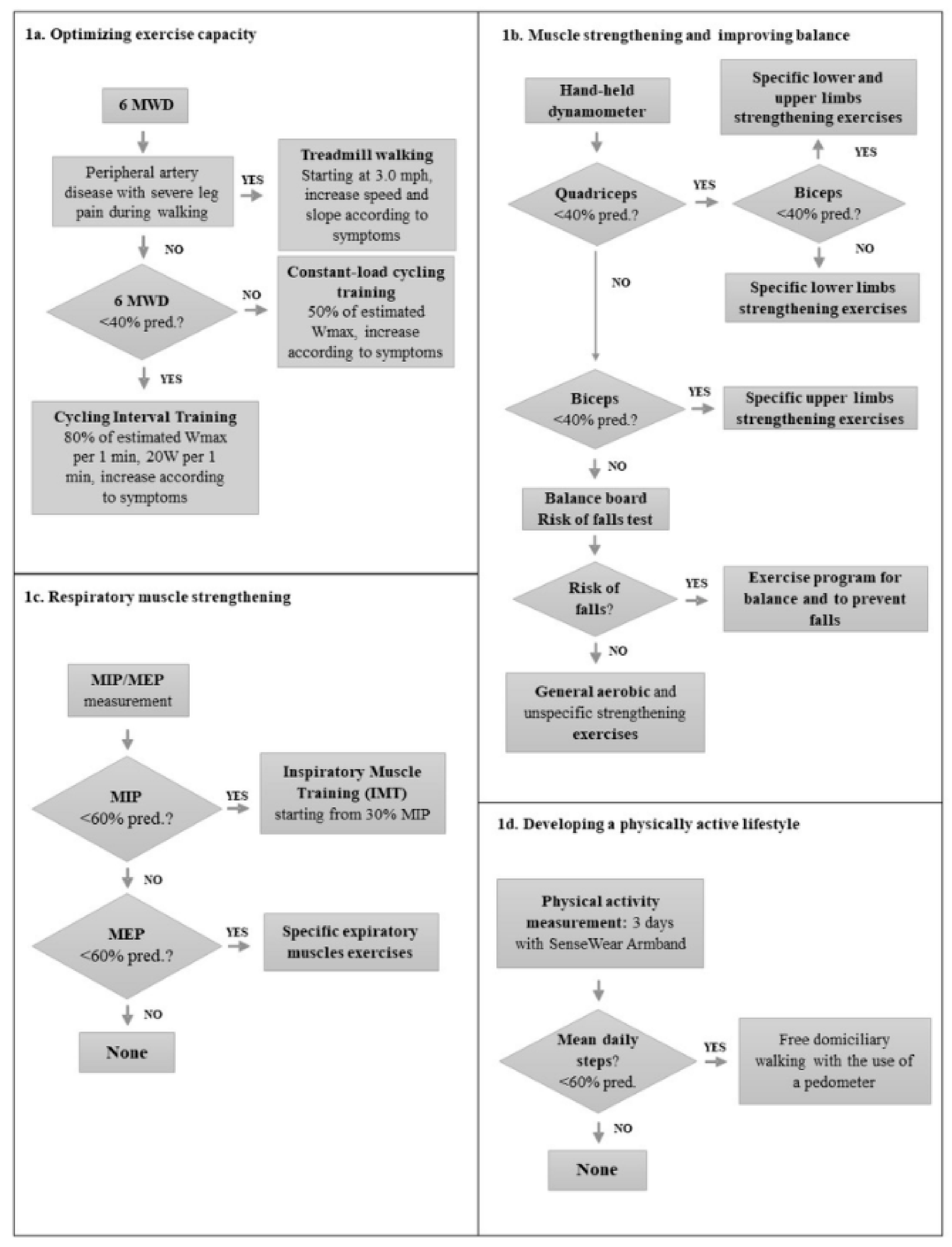
2.1. Development of the Protocol
- Stability index and risk of fall index, assessed by means of a balance board, with the predicted values of Cho et al. [24]
- Balance training including core stability training, standing on unstable surfaces, balance boards, walking on tips and heels [28]
2.2. Application of the CREMA
2.3. Feasibility and Safety
2.4. Data Management and Statistical Analysis
- Obstructive: Patients with chronic obstructive pulmonary disease (COPD) with or without chronic respiratory failure, or asthma according to the ICD9 classification. COPD was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [38], and asthma according to the Global Initiative for Asthma (GINA) criteria [39]. In patients with lung diseases, dynamic and static lung volumes were assessed according to the American Thoracic Society (ATS) guidelines [40] by means of a body plethysmograph or a water-sealed spirometer. The predicted values were those of Quanjer et al. [41].
- Restrictive: Patients with interstitial lung diseases, obesity hypoventilation syndrome, kyphoscoliosis, or mixed diagnoses with forced vital capacity (FVC) <80% of predicted and with Forced Expiratory Volume at 1 s (FEV1) /FVC > 70%.
- REVASC: Patients that have undergone a coronary artery by-pass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA) in the previous 3 months.
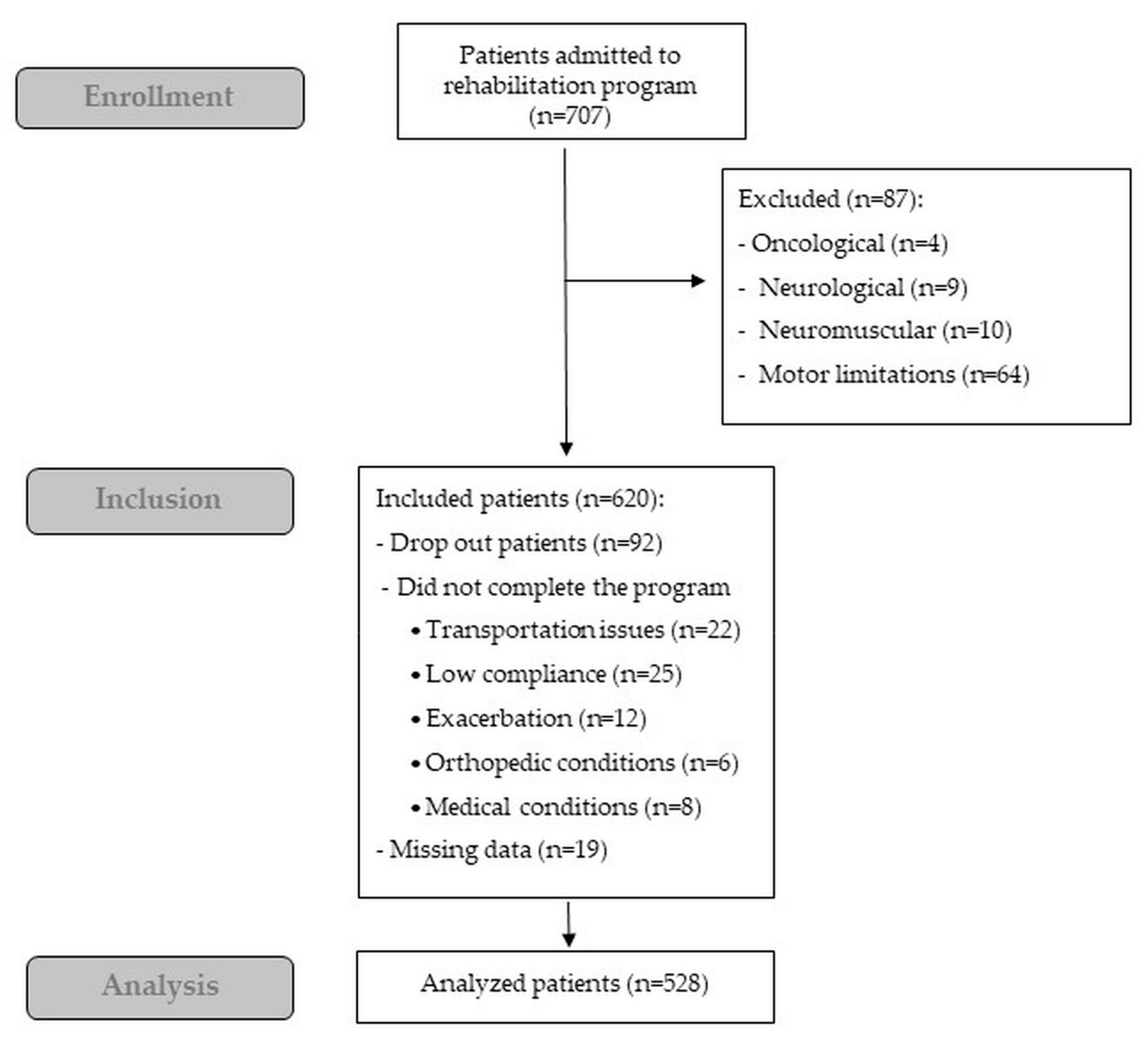
3. Results
Feasibility and Safety
4. Discussion
4.1. Limitations of the Study
4.2. Practical Implication
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, W.M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). G. Ital. Cardiol. 2013, 14, 328–392. [Google Scholar]
- Casaburi, R.; Patessio, A.; Ioli, F.; Zanaboni, S.; Donner, C.F.; Wasserman, K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am. Rev. Respir. Dis. 1991, 143, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.S.; Sagar, V.A.; Davies, E.J.; Briscoe, S.; Coats, A.J.; Dalal, H.; Lough, F.; Rees, K.; Singh, S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst. Rev. 2014, 4, CD003331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzani, A.; Hamm, L.F.; Jones, A.M.; McBride, P.E.; Moholdt, T.; Stone, J.A.; Urhausen, A.; Williams, M.A. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, 442–467. [Google Scholar]
- Piepoli, M.F.; Binno, S.; Corrà, U.; Seferovic, P.; Conraads, V.; Jaarsma, T.; Schmid, J.P.; Filippatos, G.; Ponikowski, P.P. Committee on Exercise Physiology & Training of the Heart Failure Association of the ESC. ExtraHF survey: The first European survey on implementation of exercise training in heart failure patients. Eur. J. Heart Fail. 2015, 17, 631–638. [Google Scholar]
- Vogiatzis, I.; Rochester, C.L.; Spruit, M.A.; Troosters, T.; Clini, E.M. Increasing implementation and delivery of pulmonary rehabilitation: Key messages from the new ATS/ERS policy statement. Eur. Respir. J. 2016, 47, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Maestri, R.; Bruschi, C.; Fracchia, C.; Pinna, G.D.; Fanfulla, F.; Ambrosino, N. Physiological and clinical characteristics of patients with COPD admitted to an inpatient pulmonary rehabilitation program: A real-life study. Pulmonology 2019, 25, 71–78. [Google Scholar] [CrossRef]
- Evans, R.A.; Singh, S.J.; Collier, R.; Loke, I.; Steiner, M.C.; Morgan, M.D. Generic, symptom based, exercise rehabilitation; integrating patients with COPD and heart failure. Respir. Med. 2010, 104, 1473–1481. [Google Scholar] [CrossRef] [Green Version]
- Man, W.D.; Chowdhury, F.; Taylor, R.S.; Evans, R.A.; Doherty, P.; Singh, S.J.; Booth, S.; Thomason, D.; Andrews, D.; Lee, C.; et al. Building consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure. Chron. Respir. Dis. 2016, 13, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, R.M. Therapist-Driven Protocols: New Incentives for Change. Respir. Care 2015, 60, 757–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achttien, R.J.; Vromen, T.; Staal, J.B.; Peek, N.; Spee, R.F.; Niemeijer, V.M.; Kemps, H.M.; Panel, M.E. Development of evidence-based clinical algorithms for prescription of exercise-based cardiac rehabilitation. Neth. Heart J. 2015, 23, 563–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, D.; Dendale, P.; Coninx, K.; Vanhees, L.; Piepoli, M.F.; Niebauer, J.; Cornelissen, V.; Pedretti, R.; Geurts, E.; Ruiz, G.R.; et al. The European Association of Preventive Cardiology Exercise Prescription in Everyday Practice and Rehabilitative Training (EXPERT) tool: A digital training and decision support system for optimized exercise prescription in cardiovascular disease. Concept, definitions and construction methodology. Eur. J. Prev. Cardiol. 2017, 24, 1017–1031. [Google Scholar] [PubMed]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar] [PubMed] [Green Version]
- Humphrey-Murto, S.; Varpio, L.; Wood, T.J.; Gonsalves, C.; Ufholz, L.A.; Mascioli, K.; Wang, C.; Foth, T. The use of the Delphi and other consensus group methods in medical education research: A Review. Acad. Med. 2017, 92, 1491–1498. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Chetta, A.; Zanini, A.; Pisi, G.; Aiello, M.; Tzani, P.; Neri, M.; Olivieri, D. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir. Med. 2006, 100, 1573–1578. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-min walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Andrews, A.W.; Thomas, M.W.; Bohannon, R.W. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys. Ther. 1996, 76, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, T.; Beaumont, M.; de Bisschop, C.; Bazerque, L.; Le Blanc, C.; Vincent, A.; Ouksel, H.; Chambellan, A. Determining the minimally important difference in quadriceps strength in individuals with COPD using a fixed dynamometer. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2685–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef] [PubMed]
- Black, L.F.; Hyatt, R.E. Maximal respiratory pressures: Normal values and relationship to age and sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar] [PubMed]
- Cho, K.H.; Bok, S.K.; Kim, Y.J.; Hwang, S.L. Effect of lower limb strength on falls and balance of the elderly. Ann. Rehabil. Med. 2012, 36, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.; Winnard, A.; Chynkiamis, N.; Boyle, S.; Burtin, C.; Vogiatzis, I. Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: A systematic review and meta-analysis. Eur. Respir. Rev. 2019, 28, 190039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teylan, M.; Kantorowski, A.; Homsy, D.; Kadri, R.; Richardson, C.; Moy, M. Physical activity in COPD: Minimal clinically important difference for medical events. Chron. Respir. Dis. 2019, 16, 1479973118816424. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Hakamy, A.; Bolton, C.E.; McKeever, T.M. The effect of pulmonary rehabilitation on mortality, balance, and risk of fall in stable patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 2017, 14, 54–62. [Google Scholar] [CrossRef]
- Wu, J.; Kuang, L.; Fu, L. Effects of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Congenit. Heart Dis. 2018, 13, 194–202. [Google Scholar] [CrossRef]
- Charususin, N.; Gosselink, R.; Decramer, M.; Demeyer, H.; McConnell, A.; Saey, D.; Maltais, F.; Derom, E.; Vermeersch, S.; Heijdra, Y.F.; et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax 2018, 73, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Hermes, B.M.; Cardoso, D.M.; Gomes, T.J.; Santos, T.D.; Vicente, M.S.; Pereira, S.N.; Barbosa, V.A.; Albuquerque, I.M. Short-term inspiratory muscle training potentiates the benefits of aerobic and resistance training in patients undergoing CABG in phase II cardiac rehabilitation program. Rev. Bras. Cir. Cardiovasc. 2015, 30, 474–481. [Google Scholar] [PubMed] [Green Version]
- Kendall, F.; Oliveira, J.; Peleteiro, B.; Pinho, P.; Bastos, P.T. Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: A systematic review and meta-analysis. Disabil Rehabil. 2018, 40, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Ter Hoeve, N.; Sunamura, M.; Stam, H.J.; Boersma, E.; Geleijnse, M.L.; van Domburg, R.T.; van den Berg-Emons, R.J.G. Effects of two behavioral cardiac rehabilitation interventions on physical activity: A randomized controlled trial. Int. J. Cardiol. 2018, 255, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widyastuti, K.; Makhabah, D.N.; Setijadi, A.R.; Sutanto, Y.S.; Ambrosino, N. Benefits and costs of home pedometer assisted physical activity in patients with COPD. A preliminary randomized controlled trial. Pulmonology 2018, 24, 211–218. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Maltais, F.; LeBlanc, P.; Jobin, J.; Berube, C.; Bruneau, J.; Carrier, L.; Breton, M.J.; Falardeau, G.; Belleau, R. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 155, 555–561. [Google Scholar] [CrossRef]
- Goodrich, D.E.; Larkin, A.R.; Lowery, J.C.; Holleman, R.G.; Richardson, C.R. Adverse events among high-risk participants in a home-based walking study: A descriptive study. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Global Initiative for Chronic Obstructive lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2019 Report). Available online: www.goldcopd.org (accessed on 21 November 2019).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2019. Available online: www.ginasthma.org (accessed on 15 December 2019).
- Culver, B.H.; Graham, B.L.; Coates, A.L.; Wanger, J.; Berry, C.E.; Clarke, P.K.; Hallstrand, T.S.; Hankinson, J.L.; Kaminsky, D.A.; MacIntyre, N.R.; et al. ATS Committee on Proficiency Standards for Pulmonary Function Laboratories. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am. J. Respir. Crit. Care Med. 2017, 196, 1463–1472. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [PubMed]
- Goud, R.; de Keizer, N.F.; ter Riet, G.; Wyatt, J.C.; Hasman, A.; Hellemans, I.M.; Peek, N. Effect of guideline based computerised decision support on decision making of multidisciplinary teams: Cluster randomised trial in cardiac rehabilitation. BMJ 2009, 338, b1440. [Google Scholar] [CrossRef] [Green Version]
- Ambrosino, N. Inspiratory muscle training in stable COPD patients: Enough is enough? Eur. Respir. J 2018, 51, 1702285. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Augustin, I.M.; Vanfleteren, L.E.; Janssen, D.J.; Gaffron, S.; Pennings, H.J.; Smeenk, F.; Pieters, W.; van den Bergh, J.J.; Michels, A.J.; et al. CIRO+ Rehabilitation Network. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 2015, 46, 1625–1635. [Google Scholar] [CrossRef]
- De Schutter, A.; Kachur, S.; Lavie, C.J.; Menezes, A.; Shum, K.K.; Bangalore, S.; Arena, R.; Milani, R.V. Cardiac rehabilitation fitness changes and subsequent survival. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Witvrouwen, I.; Pattyn, N.; Gevaert, A.B.; Possemiers, N.; Van Craenenbroeck, A.H.; Cornelissen, V.A.; Beckers, P.J.; Vanhees, L.; Van Craenenbroeck, E.M. Predictors of response to exercise training in patients with coronary artery disease—A subanalysis of the SAINTEX-CAD study. Eur. J. Prev. Cardiol. 2019, 26, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.P.; Zurek, M.; Saner, H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur. J. Prev. Cardiol. 2013, 20, 585–592. [Google Scholar] [CrossRef]
- Wouters, E.F.M.; Wouters, B.B.R.E.; Augustin, I.M.L.; Houben-Wilke, S.; Vanfleteren, L.E.G.W.; Franssen, F.M.E. Personalised pulmonary rehabilitation in COPD. Eur. Respir. Rev. 2018, 27, 170125. [Google Scholar] [CrossRef]
| All | Obstructive (O) | Restrictive (R) | CHD | REVASC (RE) | p-Value | |
|---|---|---|---|---|---|---|
| N (%) | 620 | 320 (51.6) | 55 (8.9) | 154 (24.8) | 91 (14.9) | |
| COPD = 205 COPD + CRF = 67 Asthma = 48 | ILD = 10 OHS = 6 Other RS = 39 | CHF = 36 CAD = 118 | PTCA = 59 CABG = 32 | |||
| Age, years | 67.1 (10.5) | 68.3 (9.0) | 65.1 (14.2) | 67.6 (9.9) | 63.0 (12.7) | <0.001 (RE vs. O <0.001; RE vs. CHD = 0.006) |
| Male, n (%) | 430 (69.3) | 224 (70) | 26 (47.3) | 109 (70.9) | 71 (78.0) | 0.001 (R vs. O = 0.006; RE vs. R <0.001; CHD vs. R = 0.012) |
| BMI, Kg/m2 | 27.9 (10.4) | 27.2 (13.5) | 27.4 (7.6) | 29.4 (5.3) | 28.0 (4.3) | 0.160 |
| FEV1, %pred | 62.5 (23.1) | 69.9 (32.9) | ||||
| FVC, %pred | 88.6 (20.5) | 72.6 (31.2) | ||||
| FEV1/FVC, % | 71.2 (20.3) | 92.1 (20.3) | ||||
| LVEF, % | 55.6 (11.3) | 57.8 (7.3) | ||||
| 6MWD, meters | 442.5 (108.6) | 434.2 (106.0) | 391.4 (110.8) | 447.3 (109.1) | 492.3 (97.2) | <0.001 (R vs. O = 0.04; RE vs. O <0.001; CHD vs. R = 0.006; RE vs. R <0.001; RE vs. CHD = 0.006) |
| 6MWD, %pred | 64.4 (14.7) | 63.2 (14.8) | 57.8 (15.8) | 65.8 (14.8) | 69.9 (11.5) | <0.001 (RE vs. O <0.001; CHD vs. R = 0.006; RE vs. R <0.001) |
| Quadriceps MVC, Kg | 30.8 (11.3) | 29.8 (10.4) | 25.7 (8.9) | 32.0 (12.4) | 35.5 (12.1) | <0.001 (RE vs. O < 0.001; CHD vs. R <0.001; RE vs. R <0.001) |
| Quadriceps MVC, %pred | 80.3 (26.1) | 79.7 (25.6) | 72.4 (27.6) | 81.0 (26.1) | 85.63 (26.6) | 0.030 (RE vs. R = 0.02) |
| Biceps MVC, Kg | 21.3 (7.5) | 20.8 (7.3) | 19.0 (7.2) | 22.1 (7.8) | 23.1 (7.2) | 0.003 (CHD vs. R = 0.04; RE vs. R = 0.006) |
| Biceps MVC, %pred | 92.4 (23.1) | 92.56 (24.4) | 90.37 (23.49) | 92.71 (22.15) | 92.54 (20.26) | 0.955 |
| MIP, cmH2O | 74.1 (24.6) | 74.2 (22.6) | 64.0 (24.1) | 74.6 (27.8) | 78.7 (24.4) | 0.006 (R vs. O = 0.02; CHD vs. R = 0.04; RE vs. R < 0.001) |
| MIP, % pred | 78.3 (24.0) | 79.4 (23.6) | 73.7 (26.8) | 77.5 (24.9) | 78.34 (22.1) | 0.426 |
| MEP, cmH2O | 103.4 (38.4) | 101.1 (34.6) | 81.8 (39.7) | 108.0 (40.7) | 116.8 (40.2) | <0.001 (R vs. O <0.001; RE vs. O = 0.006; CHD vs. R < 0.001; & RE vs R < 0.001) |
| MEP, % pred | 57.5 (18.2) | 56.76 (17.3) | 49.4 (20.2) | 59.3 (18.3) | 61.6 (18.7) | 0.006 (R vs. O = 0.04; CHD vs R = 0.006; RE vs. R < 0.001) |
| Stability index | 4.3 (2.3) | 4.3 (2.8) | 4.3 (3.6) | 4.8 (2.8) | 3.5 (1.6) | 0.008 (RE vs. CHD) = 0.006) |
| Daily steps, n/day | 5313.8 (3600.1) | 5203.0 (3626.4) | 4558.7 (2709.1) | 5452.6 (3823.5) | 5888.9 (3520.5) | 0.185 |
| All | Obstructive | Restrictive | CHD | REVASC | p-Value | |
|---|---|---|---|---|---|---|
| 6MWD < 60% of pred. | 33.2 | 36.6 | 52.7 | 29.9 | 15.4 | <0.001 (RE vs. O <0.001; CHD vs. R = 0.010; RE vs. R <0.001) |
| MVC quadriceps < 60% of pred. | 22.2 | 23.7 | 29.1 | 20.8 | 15.4 | 0.210 |
| MVC biceps < 60% of pred. | 6.77 | 6.25 | 12.73 | 5.84 | 6.59 | 0.330 |
| MIP < 60% of pred. | 21.4 | 19.1 | 32.7 | 20.1 | 25.3 | 0.174 |
| MEP < 60% of pred. | 57.3 | 58.1 | 65.4 | 55.8 | 51.6 | 0.410 |
| Risk of fall index | 36.9 | 35.3 | 36.4 | 41.6 | 35.16 | 0.592 |
| Reduced physical activity | 29.0 | 31.9 | 30.9 | 27.3 | 20.9 | 0.210 |
| All | Obstructive | Restrictive | CHD | REVASC | p-Value | |
|---|---|---|---|---|---|---|
| Constant-load cycling | 61.3 | 54.1 | 45.4 | 70.1 | 75.8 | <0.001 |
| Interval training cycling | 33.5 | 40.3 | 47.3 | 27.9 | 18.7 | <0.001 |
| Treadmill training | 4.4 | 5.0 | 5.4 | 1.9 | 5.5 | 0.410 |
| Arms endurance training | 0.7 | 1.2 | 1.8 | 0 | 0 | 0.320 |
| General strengthening | 82.1 | 81.9 | 74.5 | 83.1 | 89.0 | 0.160 |
| Selective leg strengthening | 11.3 | 13.1 | 14.5 | 12.3 | 5.5 | 0.220 |
| Selective arm strenghtening | 4.0 | 3.1 | 5.4 | 1.9 | 5.5 | 0.390 |
| Selective arm+leg strengthening | 2.5 | 1.9 | 5.4 | 2.6 | 0 | 0.160 |
| Balance training | 29.1 | 31.3 | 30.6 | 37.8 | 5.7 | <0.001 |
| Inspiratory muscle training | 20.8 | 18.4 | 27.3 | 19.6 | 26.9 | 0.197 |
| Pedometer | 27.7 | 29.7 | 30.6 | 29.4 | 15.9 | 0.067 |
| All | Obstructive | Restrictive | CHD | REVASC | p-Value | |
|---|---|---|---|---|---|---|
| 6MWD, meters % of baseline | 29.3 (59.3) 8.13 | 23.9 (60.0) * 7.03 | 29.9 (53.0) * 9.16 | 28.6 (59.6) * 8.12 | 48.7 (56.1) * 11.43 | 0.012 (RE vs. O = 0.006) |
| Quadriceps MVC, Kg % of baseline | 3.0 (8.0) * 14.0 | 2.9 (8.0) 13.6 | 4.0 (6.9) * 20.2 | 3.1 (7.5) * 13.4 | 2.8 (9.5) * 12.8 | 0.850 |
| Biceps MVC, kg % of baseline | 2.1 (4.5) * 12.0 | 1.9 (4.0) * 12.0 | 1.4 (4.9) 9.1 | 2.2 (5.1) * 11.1 | 3.1 (5.0) * 15.4 | 0.160 |
| MIP, cmH2O % of baseline | 5.8 (13.7) * 10.4 | 4.6 (12.5) * 7.9 | 0.9 (11.4)4.1 | 7.8 (15.0) * 15.6 | 9.6 (15.4) * 14.9 | 0.001 (RE vs. O = 0.030; CHD vs R = 0.020; RE vs R = 0.006) |
| MEP, cmH2O % of baseline | 8.5 (27.6) * 11.8 | 8.5 (27.4) * 12.0 | 12.6 (30.9) * 19.0 | 4.9 (26.0) * 7.2 | 12.3 (28.6) * 14.2 | 0.220 |
| Risk of fall index % of baseline | −0.6 (1.5) * −4.3 | −0.6 (1.6) * −3.6 | −0.7 (1.3) * −4.1 | −0.6 (1.5) * −6.1 | −0.4 (1.2) 4.0 | 0.900 |
| Daily steps, n/day % of baseline | −132.5 (3051.6) * −2.4 | −447.2 (2618.6) * −8.6 | −944.5 (1986.0) * −20.7 | −77.2 (3701.1) −1.4 | 1087.2 (3297.1) * 18.4 | 0.004 (RE vs. O = 0.006; RE vs R = 0.040) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonelli, C.; Vitacca, M.; Ambrosino, N.; Scalvini, S.; Rivadossi, F.; Saleri, M.; Fokom, A.G.; Speltoni, I.; Ghirardi, R.; Paneroni, M. Therapist Driven Rehabilitation Protocol for Patients with Chronic Heart and Lung Diseases: A Real-Life Study. Int. J. Environ. Res. Public Health 2020, 17, 1016. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031016
Simonelli C, Vitacca M, Ambrosino N, Scalvini S, Rivadossi F, Saleri M, Fokom AG, Speltoni I, Ghirardi R, Paneroni M. Therapist Driven Rehabilitation Protocol for Patients with Chronic Heart and Lung Diseases: A Real-Life Study. International Journal of Environmental Research and Public Health. 2020; 17(3):1016. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031016
Chicago/Turabian StyleSimonelli, Carla, Michele Vitacca, Nicolino Ambrosino, Simonetta Scalvini, Francesca Rivadossi, Manuela Saleri, Aubin G Fokom, Ilaria Speltoni, Riccardo Ghirardi, and Mara Paneroni. 2020. "Therapist Driven Rehabilitation Protocol for Patients with Chronic Heart and Lung Diseases: A Real-Life Study" International Journal of Environmental Research and Public Health 17, no. 3: 1016. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031016





