Effects of Pre-Oxidation on Haloacetonitrile and Trichloronitromethane Formation during Subsequent Chlorination of Nitrogenous Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Experimental Procedures
2.3. Analytical Methods
2.4. Determinations of Predicted Toxicity
3. Results and Discussion
3.1. HAN and TCNM Formation during Different Times of Chlorination
3.2. HAN and TCNM Formation during Pre-Oxidation Subsequent Chlorination
3.3. Proposed Formation Pathways of HAN and TCNM Formation
3.4. Effect of Bromide
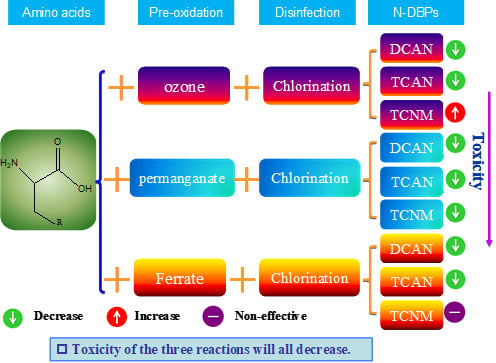
3.5. Estimated Toxicity of HANs and TCNM in Different Pre-Oxidation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Muellner, M.G.; Wagner, E.D.; K. McCalla, S.D.; Richardson, Y.; Woo, M.J. Plewa, Haloacetonitriles vs. Regulated Haloacetic Acids: Are Nitrogen-Containing DBPs More Toxic? Environ. Sci. Technol. 2007, 41, 645–651. [Google Scholar] [CrossRef]
- Ma, D.; Peng, B.; Zhang, Y.; Gao, B.; Wang, Y.; Yue, Q.; Li, Q. Influences of dissolved organic matter characteristics on trihalomethanes formation during chlorine disinfection of membrane bioreactor effluents. Bioresour. Technol. 2014, 165, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Stalter, D.; O’Malley, E.; von Gunten, U.; Escher, B.I. Fingerprinting the reactive toxicity pathways of 50 drinking water disinfection by-products. Water Res. 2016, 91, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, D.M.; Wu, Q.; Donovan, A.; Shi, H.; Ma, Y.; Jiang, H.; Wang, J. N-nitrosamine formation by monochloramine, free chlorine, and peracetic acid disinfection with presence of amine precursors in drinking water system. Chemosphere 2016, 153, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Huang, J.; Templeton, M.R.; Graham, N. Occurrence and control of nitrogenous disinfection by-products in drinking water–A review. Water Res. 2011, 45, 4341–4354. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Mitch, W.A.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Yao, D.; Deng, Y.; Sui, M.; Gao, N. Production of trihalomethanes, haloacetaldehydes and haloacetonitriles during chlorination of microcystin-LR and impacts of pre-oxidation on their formation. J. Hazard. Mater. 2017, 327, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ling, L.; Shang, C.; Guan, Y.; Fang, J. Degradation kinetics and pathways of haloacetonitriles by the UV/persulfate process. Chem. Eng. J. 2017, 320, 478–484. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Xu, X.; Wang, Z. Formation of known and unknown disinfection by-products from natural organic matter fractions during chlorination, chloramination, and ozonation. Sci. Total Environ. 2017, 587–588, 177–184. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Bai, X.; Chang, W.; Cui, C.; Zhao, W. Surface modification of smithsonite with ammonia to enhance the formation of sulfidization products and its response to flotation. Miner. Eng. 2019, 137, 1–9. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, D.; Feng, Q.; Wen, S.; Chang, W. DFT insights into the electronic properties and adsorption mechanism of HS− on smithsonite (101) surface. Miner. Eng. 2019, 141, 105846. [Google Scholar] [CrossRef]
- Chen, B.; Westerhoff, P. Predicting disinfection by-product formation potential in water. Water Res. 2010, 13, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, X.; Bai, W.; Yang, H.; Xie, Y.F. Azo compound degradation kinetics and halonitromethane formation kinetics during chlorination. Chemosphere 2017, 174, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Mccurry, D.L.; Quay, A.N.; Mitch, W.A. Ozone Promotes Chloropicrin Formation by Oxidizing Amines to Nitro Compounds. Environ. Sci. Technol. 2016, 50, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Henriet, O.; Goslan, E.H.; Parsons, S.A.; Jefferson, B. Disinfection byproduct formation and fractionation behavior of natural organic matter surrogates. Environ. Sci. Technol. 2009, 43, 5982–5989. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Fan, C.H.; Shang, C.; Quan, Z. Nitrogenous disinfection byproducts formation and nitrogen origin exploration during chloramination of nitrogenous organic compounds. Water Res. 2010, 44, 2691–2702. [Google Scholar]
- Chu, W.; Li, D.; Gao, N.; Yin, D.; Zhang, Y.; Zhu, Y. Comparison of free amino acids and short oligopeptides for the formation of trihalomethanes and haloacetonitriles during chlorination: Effect of peptide bond and pre-oxidation. Chem. Eng. J. 2015, 281, 623–631. [Google Scholar] [CrossRef]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of nitrogenous disinfection by-products in drinking water—A critical review and analysis. J. Hazard. Mater. 2012, 235–236, 1–16. [Google Scholar] [CrossRef]
- Selbes, M.; Shan, J.; Bekaroglu, S.S.K.; Karanfl, T.; Karanfl, A.T. Carbonaceous and Nitrogenous Disinfecion By-Product Formation Potentials of Amino Acids, Recent Advances in Disinfection By-Products; Chemical Society: Washington, DC, USA, 2015; pp. 215–234. [Google Scholar]
- Chu, W.; Gao, N.; Krasner, S.W.; Templeton, M.R.; Yin, D. Formation of halogenated C-, N-DBPs from chlor(am)ination and UV irradiation of tyrosine in drinking water. Environ. Pollut. 2012, 161, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Jia, A.; Wu, C.; Duan, Y. Precursors and factors affecting formation of haloacetonitriles and chloropicrin during chlor(am)ination of nitrogenous organic compounds in drinking water. J. Hazard. Mater. 2016, 308, 411–418. [Google Scholar] [CrossRef]
- Yang, X.; Shen, Q.; Guo, W.; Peng, J.; Liang, Y. Precursors and nitrogen origins of trichloronitromethane and dichloroacetonitrile during chlorination/chloramination. Chemosphere 2012, 88, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, X.; Ma, J.; Shang, C.; Zhao, Q. Characterization of algal organic matter and formation of DBPs from chlor(am)ination. Water Res. 2010, 44, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Templeton, M.R.; Rifai, O.; Ali, H.; Graham, N.J.D. Chlorinated and nitrogenous disinfection by-product formation from ozonation and post-chlorination of natural organic matter surrogates. Chemosphere 2014, 111, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.; Chang, E.E.; Chuang, C.; Liang, C.; Huang, C. Evaluating and elucidating the formation of nitrogen-contained disinfection by-products during pre-ozonation and chlorination. Chemosphere 2010, 80, 327–333. [Google Scholar] [CrossRef]
- Chu, W.; Gao, N.; Yin, D.; Deng, Y.; Templeton, M.R. Ozone-biological activated carbon integrated treatment for removal of precursors of halogenated nitrogenous disinfection by-products. Chemosphere 2011, 86, 1087–1091. [Google Scholar] [CrossRef]
- Chu, W.; Li, C.; Gao, N.; Templeton, M.R.; Zhang, Y. Terminating pre-ozonation prior to biological activated carbon filtration results in increased formation of nitrogenous disinfection by-products upon subsequent chlorination. Chemosphere 2015, 121, 33–38. [Google Scholar] [CrossRef]
- Chu, W.; Gao, N.; Deng, Y.; Templeton, M.R.; Yin, D. Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Bioresour. Technol. 2011, 102, 11161–11166. [Google Scholar] [CrossRef]
- Sharma, V.K.; Zboril, R.; Varma, R.S. Ferrates: greener oxidants with multimodal action in water treatment technologies. Acc. Chem. Res. 2015, 48, 182–191. [Google Scholar] [CrossRef]
- Gan, W.; Sharma, V.K.; Zhang, X.; Yang, L.; Yang, X. Investigation of disinfection byproducts formation in ferrate(VI) pre-oxidation of NOM and its model compounds followed by chlorination. J. Hazard. Mater. 2015, 292, 197–204. [Google Scholar] [CrossRef]
- Plewa, M.J.; Elizabeth, A.; Wagner, D.; Jazwierska, P.; And, S.D.R.; Chen, P.H.; McKague, A.B. Halonitromethane Drinking Water Disinfection Byproducts: Chemical Characterization and Mammalian Cell Cytotoxicity and Genotoxicity. Environ. Sci. Technol. 2004, 38, 62–68. [Google Scholar] [CrossRef]
- Hong, H.C.; Wong, M.H.; Liang, Y. Amino acids as precursors of trihalomethane and haloacetic acid formation during chlorination. Arch. Environ. Contam. Toxicol. 2009, 56, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, Halonitriles, Haloamides, and N-Nitrosamines: A Critical Review of Nitrogenous Disinfection Byproduct Formation Pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ram, N.M. A review of the significance and formation of chlorinated N-organic compounds in water supplies including preliminary studies on the chlorination of alanine, tryptophan, tyrosine, cytosine, and syringic acid. Environ. Int. 1985, 11, 441–451. [Google Scholar] [CrossRef]
- Goslan, E.H.; Seigle, C.; Purcell, D.; Henderson, R.; Parsons, S.A.; Jefferson, B.; Judd, S.J. Carbonaceous and nitrogenous disinfection by-product formation from algal organic matter. Chemosphere 2017, 170, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croue, J.P.; Reckhow, D.A. Destruction of chlorination byproducts with sulfite. Environ. Sci. Technol. 1989, 23, 1412–1419. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Tao, H.; Xu, H.; Gu, Y.; Chen, Z.; Yu, J. Factors affecting the formation of nitrogenous disinfection by-products during chlorination of aspartic acid in drinking water. Sci. Total Environ. 2017, 575, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Bond, T.; Mokhtar Kamal, N.H.; Bonnisseau, T.; Templeton, M.R. Disinfection by-product formation from the chlorination and chloramination of amines. J. Hazard. Mater. 2014, 278, 288–296. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Fang, J.; Guan, Y.; Yue, S.; Li, X.; Chen, L. Comparison of permanganate preoxidation and preozonation on algae containing water: cell integrity, characteristics, and chlorinated disinfection byproduct formation. Environ. Sci. Technol. 2013, 47, 14051–14061. [Google Scholar] [CrossRef]
- JQ, J.; B, L. Progress in the development and use of ferrate (VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res. 2002, 36, 1397–1408. [Google Scholar]
- Pang, S.Y.; Jiang, J.; Gao, Y.; Zhou, Y.; Huangfu, X.; Liu, Y.; Ma, J. Oxidation of Flame Retardant Tetrabromobisphenol A by Aqueous Permanganate: Reaction Kinetics, Brominated Products, and Pathways. Environ. Sci. Technol. 2014, 48, 615–623. [Google Scholar] [CrossRef]
- von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Yang, X.; Guo, W.; Lee, W. Formation of disinfection byproducts upon chlorine dioxide preoxidation followed by chlorination or chloramination of natural organic matter. Chemosphere 2013, 91, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wang, Q.; Chu, W.; Templeton, M.R. The impact of changes in source water quality on trihalomethane and haloacetonitrile formation in chlorinated drinking water. Chemosphere 2014, 117, 251–255. [Google Scholar] [CrossRef] [PubMed]





| Polarity | Type | Amino Acid | Structure | pK1 | pK2 | pK3 | Designation * |
|---|---|---|---|---|---|---|---|
| Non-Polar | Tryptophane |  | 2.5 | 9.4 | - | L | |
| Polar | Neutral | Tyrosine |  | 2.2 | 9.2 | 10.5 | W |
| Acidic | Aspartic Acid |  | 2.0 | 10.0 | 4.04 | W | |
| Basic | Histidine |  | 1.8 | 9.3 | 6.8 | W |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Lin, C.; Shen, Z.; Liu, Z.; Xu, H.; Cheng, J.; Wen, X. Effects of Pre-Oxidation on Haloacetonitrile and Trichloronitromethane Formation during Subsequent Chlorination of Nitrogenous Organic Compounds. Int. J. Environ. Res. Public Health 2020, 17, 1046. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031046
Wang A, Lin C, Shen Z, Liu Z, Xu H, Cheng J, Wen X. Effects of Pre-Oxidation on Haloacetonitrile and Trichloronitromethane Formation during Subsequent Chlorination of Nitrogenous Organic Compounds. International Journal of Environmental Research and Public Health. 2020; 17(3):1046. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031046
Chicago/Turabian StyleWang, Ao, Chenshuo Lin, Zhen Shen, Zhigang Liu, Hang Xu, Jiapei Cheng, and Xin Wen. 2020. "Effects of Pre-Oxidation on Haloacetonitrile and Trichloronitromethane Formation during Subsequent Chlorination of Nitrogenous Organic Compounds" International Journal of Environmental Research and Public Health 17, no. 3: 1046. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031046