Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site
2.2. Sample Collection
2.3. Determination of Physico-Chemical Parameters
2.4. Heavy Metal Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Industrial Wastewater Physicochemical Characteristics
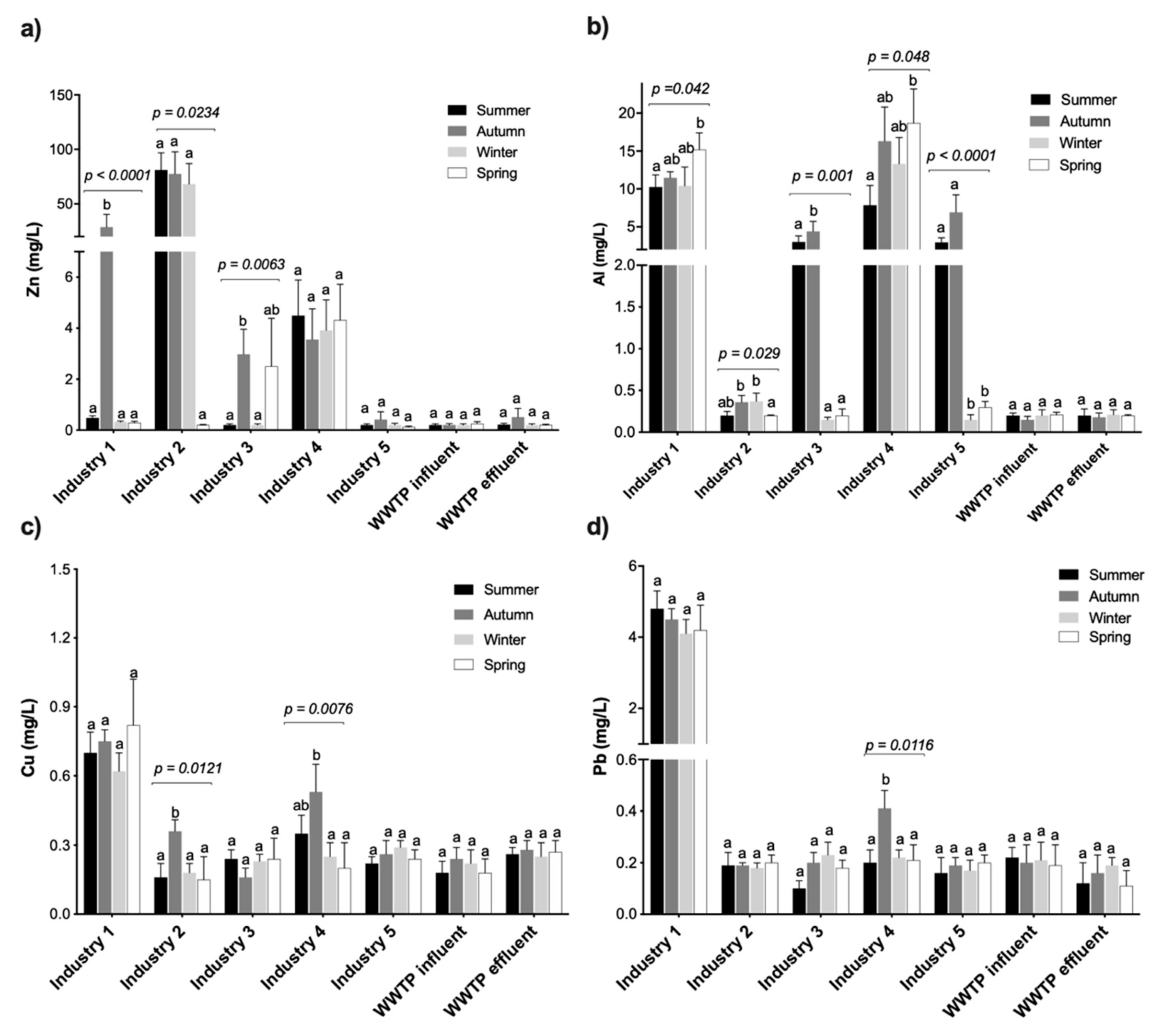
3.2. Heavy Metal Composition of Industrial Wastewaters
3.3. Impact of Industrial Wastewater and WWTP Effluents on Vaal River
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meyer, A.M.; Klein, C.; Fünfrocken, E.; Kautenburger, R.; Beck, H.P. Real-time monitoring of water quality to identify pollution pathways in small and middle scale rivers. Sci. Total Environ. 2019, 651, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Kamika, I.; Momba, M.N.B. Assessing the resistance and bioremediation ability of selected bacterial and protozoan species to heavy metals in metal-rich industrial wastewater. BMC Microbiol. 2013, 13, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetty, S.; Pillay, L. Assessing the influence of human activities on river health: A case for two South African rivers with differing pollutant sources. Environ. Monit. Assess. 2019, 191, 168. [Google Scholar] [CrossRef] [PubMed]
- Ololade, O.O. Understanding the nexus between energy and water: A basis for human survival in South Africa. Dev. S. Afr. 2018, 35, 194–209. [Google Scholar] [CrossRef]
- Oruko, R.O.; Selvarajan, R.; Ogola, H.J.O.; Edokpayi, J.N.; Odiyo, J.O. Contemporary and future direction of chromium tanning and management in sub Saharan Africa tanneries. Process Saf. Environ. Prot. 2019. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bestetti, M.; Crotti, B.M.; Carnevale Miino, M. Electrolytic Recovery of Nickel and Copper from Acid Pickling Solutions Used to Treat Metal Surfaces. Water Air Soil Pollut. 2019, 230, 101. [Google Scholar] [CrossRef]
- Sorensen, J.P.R.; Lapworth, D.J.; Nkhuwa, D.C.W.; Stuart, M.E.; Gooddy, D.C.; Bell, R.A.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater sources in Africa. Water Res. 2015, 72, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Target quantification of azole antifungals and retrospective screening of other emerging pollutants in wastewater effluent using UHPLC –QTOF-MS. Environ. Pollut. 2019, 253, 655–666. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Whitehead, P.; Bussi, G.; Hossain, M.A.; Dolk, M.; Das, P.; Comber, S.; Peters, R.; Charles, K.J.; Hope, R.; Hossain, M.S. Restoring water quality in the polluted Turag-Tongi-Balu river system, Dhaka: Modelling nutrient and total coliform intervention strategies. Sci. Total Environ. 2018, 631, 223–232. [Google Scholar] [CrossRef]
- Sibanda, T.; Selvarajan, R.; Tekere, M. Urban effluent discharges as causes of public and environmental health concerns in South Africa’s aquatic milieu. Environ. Sci. Pollut. Res. 2015, 22, 18301–18317. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- DWS. Directorate Water Resource Planning Systems: Water Quality Planning. Resource Directed Management of Water Quality. Planning Level Review of Water Quality in South Africa. Sub-series No.WQP 2.0; Department of Water Affairs: Pretoria, South Africa, 2011.
- Chokwe, T.B.; Magubane, M.N.; Abafe, O.A.; Okonkwo, J.O.; Sibiya, I.V. Levels, distributions, and ecological risk assessments of polybrominated diphenyl ethers and alternative flame retardants in river sediments from Vaal River, South Africa. Environ. Sci. Pollut. Res. 2019, 26, 7156–7163. [Google Scholar] [CrossRef] [PubMed]
- Morrison, G.; Fatoki, O.S.; Persson, L.; Ekberg, A. Assessment of the impact of point source pollution from the Keiskammahoek Sewage Treatment Plant on the Keiskamma River-pH, electrical conductivity, oxygen-demanding substance (COD) and nutrients. Water SA 2001, 27, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Momba, M.; Osode, A.; Sibewu, M. The impact of inadequate wastewater treatment on the receiving water bodies–Case study: Buffalo City and Nkokonbe Municipalities of the Eastern Cape Province. Water SA 2006, 32. [Google Scholar] [CrossRef] [Green Version]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of Wastewater on Surface Water Quality in Developing Countries: A Case Study of South Africa; Water Quality INTECH: Vienna, Austria, 2017; pp. 401–416. [Google Scholar]
- Ntuli, F. Management and Control of Industrial Effluents Discharged to Public Sewers: A Case Study. Int. J. Environ. Ecol. Eng. 2012, 6, 469–474. [Google Scholar]
- Mema, V. Impact of poorly maintained waste water and sewage treatment plants: Lessons from South Africa. Resource 2010, 12, 60–61. [Google Scholar]
- GIBB. The Sedibeng Regional Sanitation Scheme (SRSS): Leeuwkuil WWTW WML—Draft Scoping Report; Organisation Undoing Tax Abuse, OUTA: Johannesburg, South Africa, 2017.
- Sedibeng District Municipality. Southern Corridor Implementation Plan; Sedibeng District Municipality: Johannesburg, South Africa, 2018. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Martin, T.D.; Brockhoff, C.A.; Creed, J.T.; Long, S.E. Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. Methods Determ. Met. Environ. Samples 1992, 33–91. [Google Scholar]
- Johnston, J.; LaPara, T.; Behrens, S. Composition and Dynamics of the Activated Sludge Microbiome during Seasonal Nitrification Failure. Sci. Rep. 2019, 9, 4565. [Google Scholar] [CrossRef] [Green Version]
- Comber, S.D.W.; Gardner, M.J.; Ellor, B. Seasonal variation of contaminant concentrations in wastewater treatment works effluents and river waters. Environ. Technol. 2019, 1–15. [Google Scholar] [CrossRef]
- Mahgoub, S.; Samaras, P.; Abdelbasit, H.; Abdelfattah, H. Seasonal variation in microbiological and physicochemical characteristics of municipal wastewater in Al-Sharqiya province, Egypt (case study). Desalin. Water Treat. 2016, 57, 2355–2364. [Google Scholar] [CrossRef]
- Department of Water Affairs and Forestry. Revision of General Authorisations in Terms of Section 39 of the National Water Act, 1998 (Act No. 36 of 1998), No. 665; Department of Water Affairs: Pretoria, South Africa, 2013.
- Howell, C.L.; Myburgh, P.A.; Lategan, E.L.; Hoffman, J.E. Seasonal variation in composition of winery wastewater in the breede River Valley with respect to classical water quality parameters. S. Afr. J. Enol. Vitic. 2016, 37, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Cao, H.; Zhang, X.; Lin, X.; Zheng, W.; Cao, G.; Sun, Y.; Zhang, Y. Spent lead-acid battery recycling in China–A review and sustainable analyses on mass flow of lead. Waste Manag. 2017, 64, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Tekere, M.; Sibanda, T.; Maphangwa, K.W. An assessment of the physicochemical properties and toxicity potential of carwash effluents from professional carwash outlets in Gauteng Province, South Africa. Environ. Sci. Pollut. Res. Int. 2016, 23, 11876–11884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.J.; Ismail, A.F.; Firdaus, S. Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Sep. Purif. Technol. 2013, 104, 26–31. [Google Scholar] [CrossRef]
- Bayo, J.; López-Castellanos, J. Principal factor and hierarchical cluster analyses for the performance assessment of an urban wastewater treatment plant in the Southeast of Spain. Chemosphere 2016, 155, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Gerber, E.L.; Rockaway, T.D. Temporal performance assessment of wastewater treatment plants by using multivariate statistical analysis. J. Environ. Manag. 2017, 193, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kumar, A.; Maiti, S.K.; Chowdhury, A. Seasonal variation in heavy metal contaminations in water and sediments of Jamshedpur stretch of Subarnarekha river, India. Environ. Earth Sci. 2016, 75, 265. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Kefford, B.J.; Piscart, C.; Prat, N.; Schäfer, R.B.; Schulz, C.-J. Salinisation of rivers: An urgent ecological issue. Environ. Pollut. 2013, 173, 157–167. [Google Scholar]
- Adewumi, J.R.; Olanrewaju, O.O.; Adewumi, J.R.; Olanrewaju, O.O. Green Drop Certification of wastewater treatment plants in South Africa—An initiative to reduce drinking water sources pollution and bedrock of wastewater reuse. Afr. J. Sci. Technol. Innov. Dev. 2011, 3. [Google Scholar]
- Edokpayi, J.N. Assessment Of The Efficiency Of Wastewater Treatment Facilities and the Impact of Their Effluent on Surface Water and Sediments in Vhembe District, South Africa. Ph.D. Thesis, University of Venda, Thohoyandou, South Africa, 2016. [Google Scholar]
- Yildiz, B.S. Water and wastewater treatment: Biological processes. In Metropolitan Sustainability; Elsevier: Amsterdam, The Netherlands, 2012; pp. 406–428. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Chapter 11—Effluent treatment. In Principles of Fermentation Technology; Butterworth-Heinemann: Oxford, UK, 2017; pp. 687–723. ISBN 978-0-08-099953-1. [Google Scholar]
- Vu, H.H.; Gu, S.; Thriveni, T.; Khan, D.M.; Tuan, Q.L.; Ahn, W.J. Sustainable Treatment for Sulfate and Lead Removal from Battery Wastewater. Sustainbility 2019, 11, 3497. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Scheufele, F.B.; Espinoza-Quiñones, F.R.; Módenes, A.N.; Vieira, M.G.A.; Kroumov, A.D.; Borba, C.E. A comprehensive evaluation of heavy metals removal from battery industry wastewaters by applying bio-residue, mineral and commercial adsorbent materials. J. Mater. Sci. 2018, 53, 7976–7995. [Google Scholar] [CrossRef]
- Mohamed, R.; Saphira, R.M.; Kutty, A.I.; Mariam, N.; Kassim, M.; Hashim, A. Efficiency of using commercial and natural coagulants in treating car wash wastewater treatment. Aust. J. Basic Appl. Sci. 2014, 8, 227–234. [Google Scholar]
- Mazumder, D.; Mukherjee, S. Treatment of automobile service station wastewater by coagulation and activated sludge process. Int. J. Environ. Sci. Dev. 2011, 2, 64. [Google Scholar] [CrossRef]
- Ipeaiyeda, A.R.; Onianwa, P.C. Monitoring and assessment of sediment contamination with toxic heavy metals: Case study of industrial effluent dispersion in Alaro River, Nigeria. Appl. Water Sci. 2018, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Zinabu, E.; Kelderman, P.; van der Kwast, J.; Irvine, K. Monitoring river water and sediments within a changing Ethiopian catchment to support sustainable development. Environ. Monit. Assess. 2019, 191, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SANS. South African National Standard 241-1: Drinking Water. Part 1: Microbiological, Physical, Aesthetic and Chemical Determinants. 241-2:2015 Drinking Water, Part 2: Application of SANS 241-1; SANS: Bethesda, MD, USA, 2015. [Google Scholar]
- Osman, A.G.M.; Kloas, W. Water quality and heavy metal monitoring in water, sediments, and tissues of the African Catfish Clarias gariepinus (Burchell, 1822) from the River Nile, Egypt. J. Environ. Prot. 2010, 1, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Jordaan, K.; Bezuidenhout, C.C. The impact of physico-chemical water quality parameters on bacterial diversity in the Vaal River, South Africa. Water SA 2013, 39, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, A. Freshwater Challenges of South Africa and Its Upper Vaal River; Springer: Berlin, Germany, 2017; ISBN 3319495011. [Google Scholar]
- Chigor, V.N.; Sibanda, T.; Okoh, A.I. Variations in the physicochemical characteristics of the Buffalo River in the Eastern Cape Province of South Africa. Environ. Monit. Assess. 2013, 185, 8733–8747. [Google Scholar] [CrossRef] [PubMed]
- Odjadjare, E.E.; Okoh, A.I. Physicochemical quality of an urban municipal wastewater effluent and its impact on the receiving environment. Environ. Monit. Assess. 2010, 170, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, C.; Liu, Y.; Zeng, G.; Tang, X.; Dai, L.; Lan, S. Immobilizing of heavy metals in sediments contaminated by nonferrous metals smelting plant sewage with sulfate reducing bacteria and micro zero valent iron. Chem. Eng. J. 2016, 306, 393–400. [Google Scholar] [CrossRef]
- Ololade, I.A.; Lajide, L.; Ololade, O.O.; Adeyemi, O. Metal partitioning in sediment pore water from the Ondo coastal region, Nigeria. Toxicol. Environ. Chem. 2011, 93, 1098–1110. [Google Scholar] [CrossRef]
- Wepener, V.; van Dyk, C.; Bervoets, L.; O’Brien, G.; Covaci, A.; Cloete, Y. An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys. Chem. Earth Parts A/B/C 2011, 36, 949–962. [Google Scholar] [CrossRef]
- van Rensburg, S.J.; Barnard, S.; Booyens, S. Comparison of phytoplankton assemblages in two differentially polluted streams in the Middle Vaal Catchment, South Africa. S. Afr. J. Bot. 2019, 125, 234–243. [Google Scholar] [CrossRef]
- Plessl, C.; Gilbert, B.M.; Sigmund, M.F.; Theiner, S.; Avenant-Oldewage, A.; Keppler, B.K.; Jirsa, F. Mercury, silver, selenium and other trace elements in three cyprinid fish species from the Vaal Dam, South Africa, including implications for fish consumers. Sci. Total Environ. 2019, 659, 1158–1167. [Google Scholar] [CrossRef]
- USEPA. Guidance for Water Quality-Based Decisions: The TMDL Process; US Environmental Protection Agency: Washington, DC, USA, 1991.
- Shoemaker, L.; Dai, T.; Koenig, J.; Hantush, M. TMDL Model Evaluation and Research Needs; National Risk Management Research Laboratory, US Environmental Protection: Washington, DC, USA, 2005.
- Harding, W.R. A Feasibility Evaluation of the Total Maximum Daily (pollutant) Load (TMDL) Approach for Managing Eutrophication in South African Dams: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2015; ISBN 1431206733. [Google Scholar]
- Harding, W.R. The Determination of Annual Phosphorus Loading Limits for South African Dams; Water Research Commission: Pretoria, South Africa, 2008. [Google Scholar]

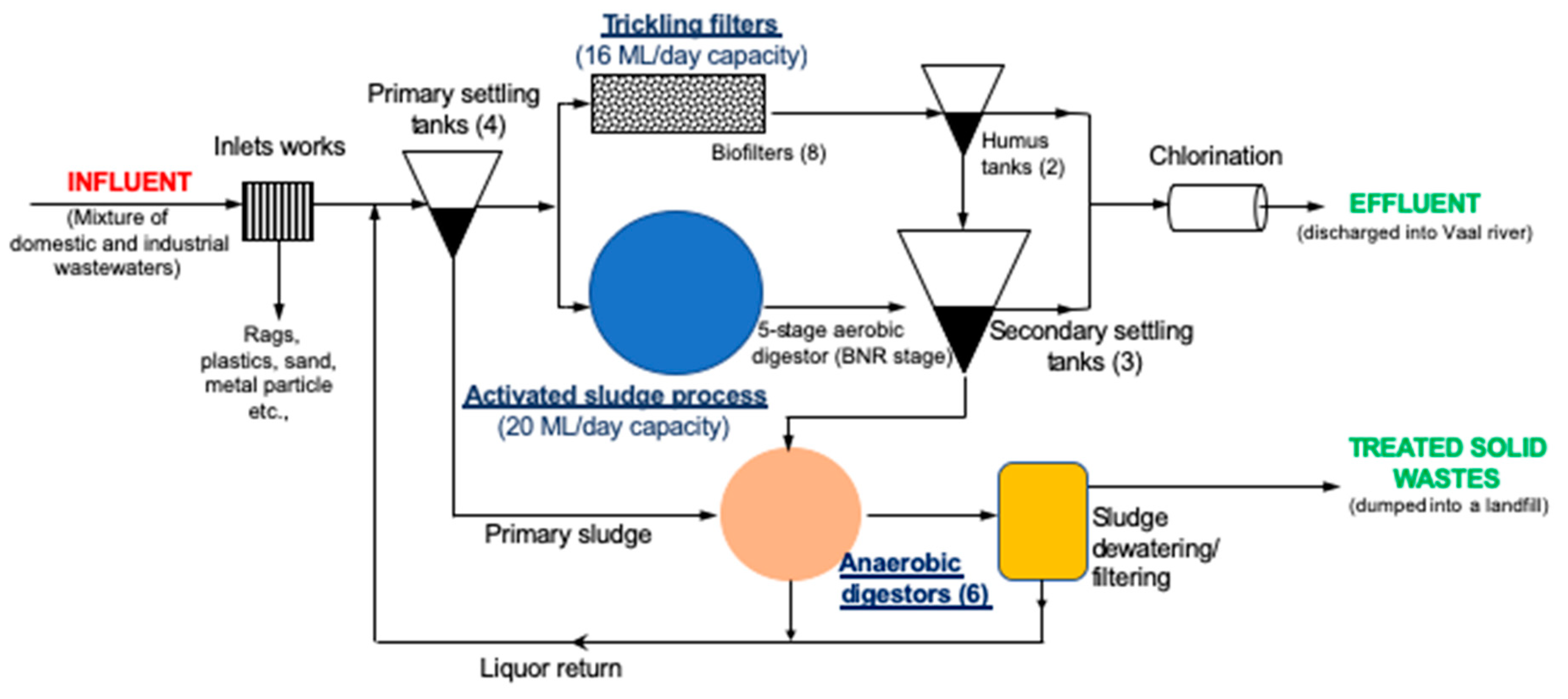
| Sampling Site | Temp (°C) | pH | BOD (mg/L) | DO (mg/L) | COD (mg/L) | TDS (mg/L) | EC (mS/cm) | Salinity (psu) |
|---|---|---|---|---|---|---|---|---|
| Industry 1: Battery | 22.2 ± 4.7 (16.4–27.4) | 4.6 ± 0.8 a (3.1–6.2) | 6.0 ± 0.6 a (5.6–6.8) | 1.6 ± 0.3 a (1.2–1.9) | 256 ± 62 a (184–320) | 1868 ± 463 b (1301–2434) | 258 ± 21 a (232–281) | 3.0 ± 2.5 ab (1.4–6.8) |
| Industry 2: Iron/metal galvanizing | 21.8 ± 4.6 (15.7–26.6) | 8.0 ± 1.1 a (6.4–8.7) | 6.0 ± 0.7 a (5.4–7.0) | 1.7 ± 0.7 ab (0.7–2.1) | 460 ± 476 abc (174–1172) | 1827 ± 489 b (1138–2223) | 2969 ± 834 ab (2223–3782) | 1.9 ± 0.5 ab (1.2–2.4) |
| Industry 3: Iron/steel | 22.3 ± 5.2 (16.2–28.2) | 9.7 ± 0.5 ab (9.2–10.2) | 5.4 ± 1.0 ac (4.2–6.6) | 1.6 ± 0.5 a ((1.0–2.2) | 1038 ± 304 c (872–1493) | 16.8 ± 4.6 a (12–22) | 30.8 ± 14.0 a (14–45) | 20.2 ± 4.9 c (14.4–24.8) |
| Industry 4: Tanking/car wash | 22.2 ± 5.1 (15.8–27.7) | 10.9 ± 2.2 b (7.7–12.5) | 5.9 ± 0.8 a (5.1–6.9) | 2.0 ± 0.2 ab (1.8–2.1) | 918 ± 294 bc (620–1209) | 2330 ± 700 b (1417–3117) | 2460 ± 1492 ab (1417–4673) | 1.4 ± 0.4 ab (0.9–2.5) |
| Industry 5: Iron/steel | 22.3 ± 5.1 (16.0–27.9) | 11.1 ± 0.9 b (10.3–12.2) | 5.9 ± 0.8 a (5.2–6.7) | 1.2 ± 0.5 a (0.8–1.9) | 310 ± 251 a (95–670) | 3654 ± 891 c (2468–4611) | 3849 ± 3756 b (1109–9224) | 5.8 ± 0.5 b (5.2–6.2) |
| WWTP influent | 21.6 ± 4.0 (16.6–26.3) | 8.3 ± 0.6 a (7.8–9.0) | 3.3 ± 1.2 b (2.1–4.9) | 1.4 ± 0.3 a (1.0–1.7) | 434 ± 50 ab (360–468) | 335 ± 14 a (321–354) | 590 ± 171 ab (337–710) | 0.34 ± 0 a (0.33–0.35) |
| WWTP effluent | 22.7 ± 5.0 (16.7–27.0) | 8.0 ± 0.3 a (7.7–8.4) | 3.8 ± 0.7 bc (3.1–4.4) | 2.7 ± 0.4 b (2.2–3.0) | 36 ± 67 a (0–136) | 258 ± 21 a (232–281) | 469 ± 143 ab (281–627) | 0.26 ± 0.02 a (0.2–0.3) |
| F-statisticτ | 0.02 | 8.06 *** | 7.32 ** | 5.05 ** | 7.61 *** | 28.63 *** | 3.88 ** | 44.38 *** |
| Green drop Standards # | 30 | 5.5–9.5 | - | - | 75 | 25 | 150 | - |
| DWAF 2013 § | - | 5.5–9.5 | - | - | 75 | 25 | 70–150 | - |
| Sampling Asite | Al | Zn | Cu | Pb |
|---|---|---|---|---|
| Industry 1: Battery | 11.8 ± 1.16 a (10.3–15.2) | 7.41 ± 2.04 (0.5–28.5) | 0.72 ± 0.04 a (0.62–0.81) | 4.64 ± 0.17 a (4.3–5.0) |
| Industry 2: Iron/metal galvanizing | 0.28 ± 0.05 b (0.2–0.37) | 56.7 ± 19.03 a (0.2–81.1) | 0.24 ± 0.04 (0.15–0.35) | 0.18 ± 0.02 (0.15–0.23) |
| Industry 3: Iron/steel | 1.95 ± 1.05 b (0.2–4.41) | 1.47 ± 0.74 (0.2–2.97) | 0.19 ± 0.02 (0.12–0.25) | 0.18 ± 0.03 (0.1–0.26) |
| Industry 4: Tanking/car wash | 14.0 ± 2.34 a (7.85–18.7) | 4.07 ± 0.21 (3.55–4.49) | 0.18 ± 0.02 (0.13–0.24) | 0.19 ± 0.03 (0.13–0.25) |
| Industry 5: Iron/steel | 2.56 ± 1.59 b (0.12–6.92) | 0.26 ± 0.05 (0.20–0.41) | 0.19 ± 0.02 (0.14–0.23) | 0.21 ± 0.00 (0.2–0.21) |
| WWTP influent | 0.18 ± 0.03 b (0.15–0.24) | 0.20 ± 0.01 (0.18–0.23) | 0.22 ± 0.02 (0.18–0.26) | 0.20 ± 0.03 (0.12–0.23) |
| WWTP effluent | 0.20 ± 0.02 b (0.15–0.24) | 0.16 ± 0.03 (0.09–0.23) | 0.18 ± 0.02 (0.14–0.24) | 0.19 ± 0.03 (0.12–0.23) |
| F–statisticτ | 23.42 *** | 7.31 *** | 46.58 *** | 584.45 *** |
| DWAF 2013 § | - | 0.1 | 0.01 | 0.01 |
| Parameter | pH | BOD | COD | DO | TDS | EC | Salinity | Al | Zn | Cu | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||
| BOD | 0.12 | 1 | |||||||||
| DO | −0.02 | −0.47 * | 1 | ||||||||
| COD | 0.19 | 0.00 | −0.06 | 1 | |||||||
| TDS | 0.25 | 0.10 | 0.16 | −0.51 * | 1 | ||||||
| EC | 0.41 | 0.04 | 0.13 | −0.29 | 0.66 ** | 1 | |||||
| Salinity | 0.14 | −0.31 | −0.13 | 0.36 | −0.60 ** | −0.36 | 1 | ||||
| Al | −0.03 | 0.25 | 0.17 | 0.10 | 0.08 | −0.20 | −0.35 | 1 | |||
| Zn | −0.24 | 0.10 | −0.01 | −0.05 | 0.05 | 0.22 | −0.25 | −0.32 | 1 | ||
| Cu | −0.61 ** | 0.09 | −0.10 | −0.35 | −0.03 | −0.37 | −0.26 | 0.41 | 0.02 | 1 | |
| Pb | −0.57 ** | 0.10 | −0.04 | −0.41 | −0.02 | −0.37 | −0.23 | 0.46 * | −0.12 | 0.96 *** | 1 |
| Parameter | pH | BOD | DO | COD | TDS | EC | Salinity | Al | Zn | Cu | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||
| BOD | 0.43 | 1 | |||||||||
| DO | −0.26 | 0.31 | 1 | ||||||||
| COD | 0.45 | −0.19 | −0.95 *** | 1 | |||||||
| TDS | 0.35 | −0.17 | −0.87 ** | 0.92 ** | 1 | ||||||
| EC | −0.21 | −0.05 | −0.12 | 0.23 | 0.66 ** | 1 | |||||
| Salinity | 0.36 | −0.22 | −0.90 ** | −0.95 *** | 0.99 *** | 0.31 | 1 | ||||
| Al | −0.36 | −0.03 | 0.53 | −0.45 | −0.14 | 0.20 | −0.21 | 1 | |||
| Zn | −0.07 | 0.24 | −0.55 | 0.44 | 0.39 | −0.05 | 0.40 | −0.29 | 1 | ||
| Cu | 0.22 | 0.16 | −0.67 | 0.56 | 0.35 | 0.00 | 0.40 | −0.88 ** | 0.63 | 1 | |
| Pb | 0.56 | −0.24 | −0.11 | 0.20 | 0.08 | −0.23 | 0.09 | −0.37 | 0.64 | 0.01 | 1 |
| Parameter * | Upstream | Downstream | Potable Water 1 | Potable Water 2 | p-Value | SANS 241-1 2015 § | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |||
| pH | 8.4 ± 0.45 | 7.8–8.8 | 8.5 ± 0.57 | 7.8–9.2 | 8.8 ± 0.36 | 8.5–9.2 | 8.9 ± 0.25 | 8.7–9.2 | 0.3521 | 5.0–9.7 |
| BOD (mg/L) | 4.497 ± 0.334 a | 4.1–4.9 | 4.305 ± 0.258 a | 4.–4.7 | 1.01 ± 0.142 b | 0.9–1.2 | 1.09 ± 0.553 b | 0.6–1.8 | <0.0001 | 5 |
| DO (mg/L) | 3.548 ± 0.403 ab | 3.1–3.9 | 3.202 ± 0.708 a | 2.2–3.8 | 4.948 ± 0.618 c | 4.5–5.8 | 4.840 ± 0.810 bc | 4.1–6.0 | 0.0045 | 75 |
| COD (mg/L) | 88 ± 57.2 a | 83–155 | 162 ± 54.6 b | 105–230 | 7.75 ± 9.18 c | 0–18 | 10.28 ± 14.03 c | 0–31 | 0.0004 | 30 |
| TDS (mg/L) | 313 ± 38 a | 312–358 | 614 ± 174 b | 381–782 | 59 ± 24 c | 30–89 | 64 ± 25 c | 27–78 | <0.0001 | - |
| EC (mS/cm) | 578 ± 160 a | 377–736 | 553 ± 130 a | 358–630 | 160 ± 78 b | 77–261 | 154 ± 80 b | 75–262 | 0.0002 | 250 |
| Salinity (psu) | 0.313 ± 0.033 a | 0.3–0.4 | 0.344 ± 0.033 a | 0.2–0.5 | 0.090 ± 0.027 b | 0.07–0.13 | 0.153 ± 0.127 b | 0.07–0.34 | 0.0005 | - |
| Al (mg/L) | 0.165 ± 0.044 b | 0.11–0.2 | 0.218 ± 0.092 ab | 0.14–0.35 | 0.078 ± 0.049 a | 0.012–0.13 | 0.060 ± 0.062 a | 0.05–0.15 | 0.0146 | 0.3 |
| Zn (mg/L) | 0.220 ± 0.091 | 0.12–0.34 | 0.380 ± 0.269 | 0.2–0.77 | 0.234 ± 0.047 | 0.2–0.3 | 0.310 ± 0.220 | 0.2–0.64 | 0.5541 | 5 |
| Cu (mg/L) | 0.119 ± 0.076 a | 0.03–0.21 | 0.166 ± 0.123 b | 0.01–0.28 | <MDL α | <MDL | 0.0138 | 2 | ||
| Pb (mg/L) | 0.098 ± 0.033 a | 0.05-0.13 | 0.183 ± 0.041 b | 0.13-0.21 | <MDL | <MDL | <0.0001 | 0.01 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iloms, E.; Ololade, O.O.; Ogola, H.J.O.; Selvarajan, R. Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1096. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031096
Iloms E, Ololade OO, Ogola HJO, Selvarajan R. Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa. International Journal of Environmental Research and Public Health. 2020; 17(3):1096. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031096
Chicago/Turabian StyleIloms, Eunice, Olusola O. Ololade, Henry J. O. Ogola, and Ramganesh Selvarajan. 2020. "Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa" International Journal of Environmental Research and Public Health 17, no. 3: 1096. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031096







