Quantifying the Health Burden Misclassification from the Use of Different PM2.5 Exposure Tier Models: A Case Study of London
Abstract
:1. Introduction
2. Materials and Methods
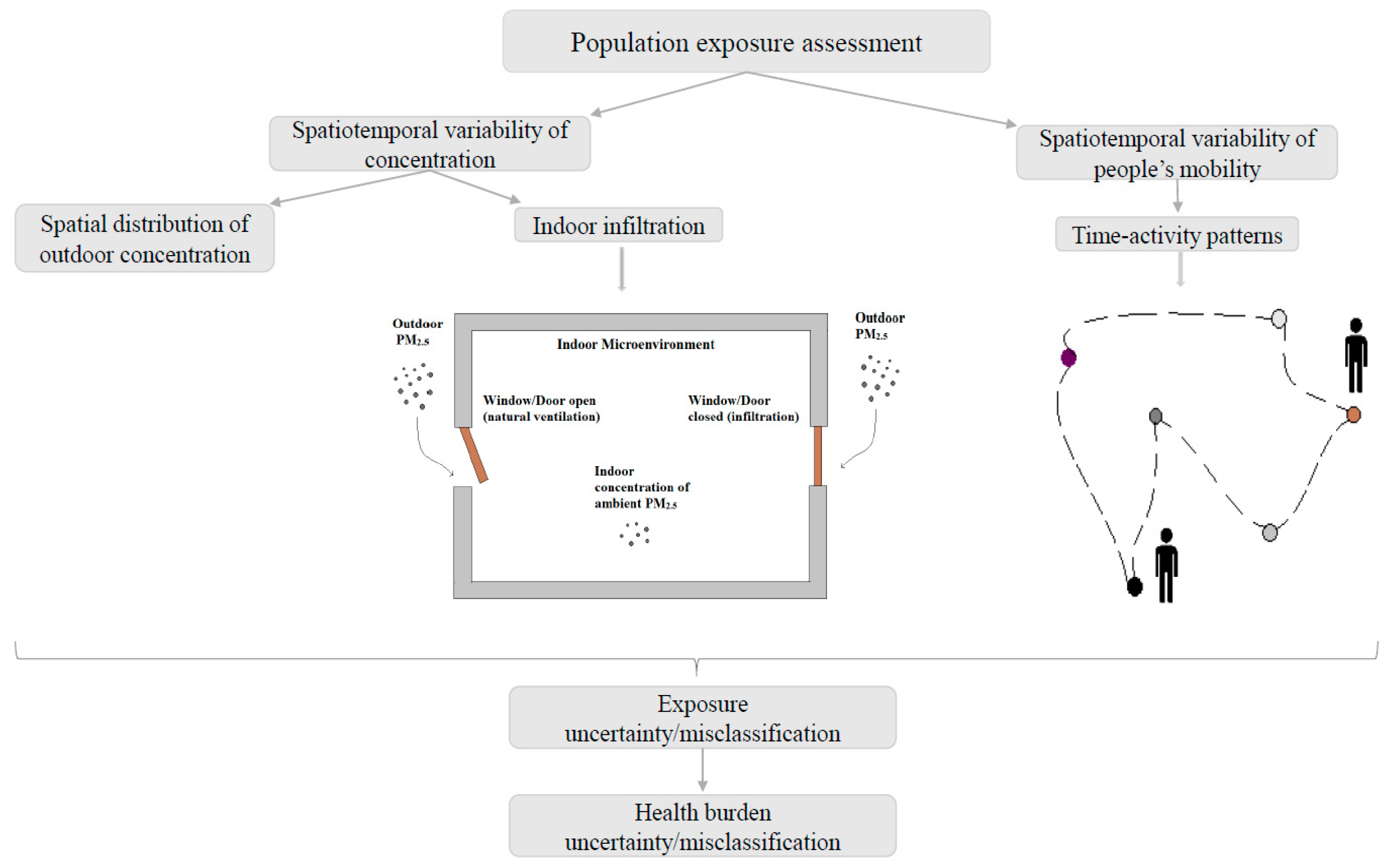
2.1. Developing Tier Models to Estimate Human Exposure
PM2.5 Concentration in the London Underground
2.2. Simulating PM2.5 Exposure Concentration and Estimating Health Impact Using BenMap-CE
3. Results
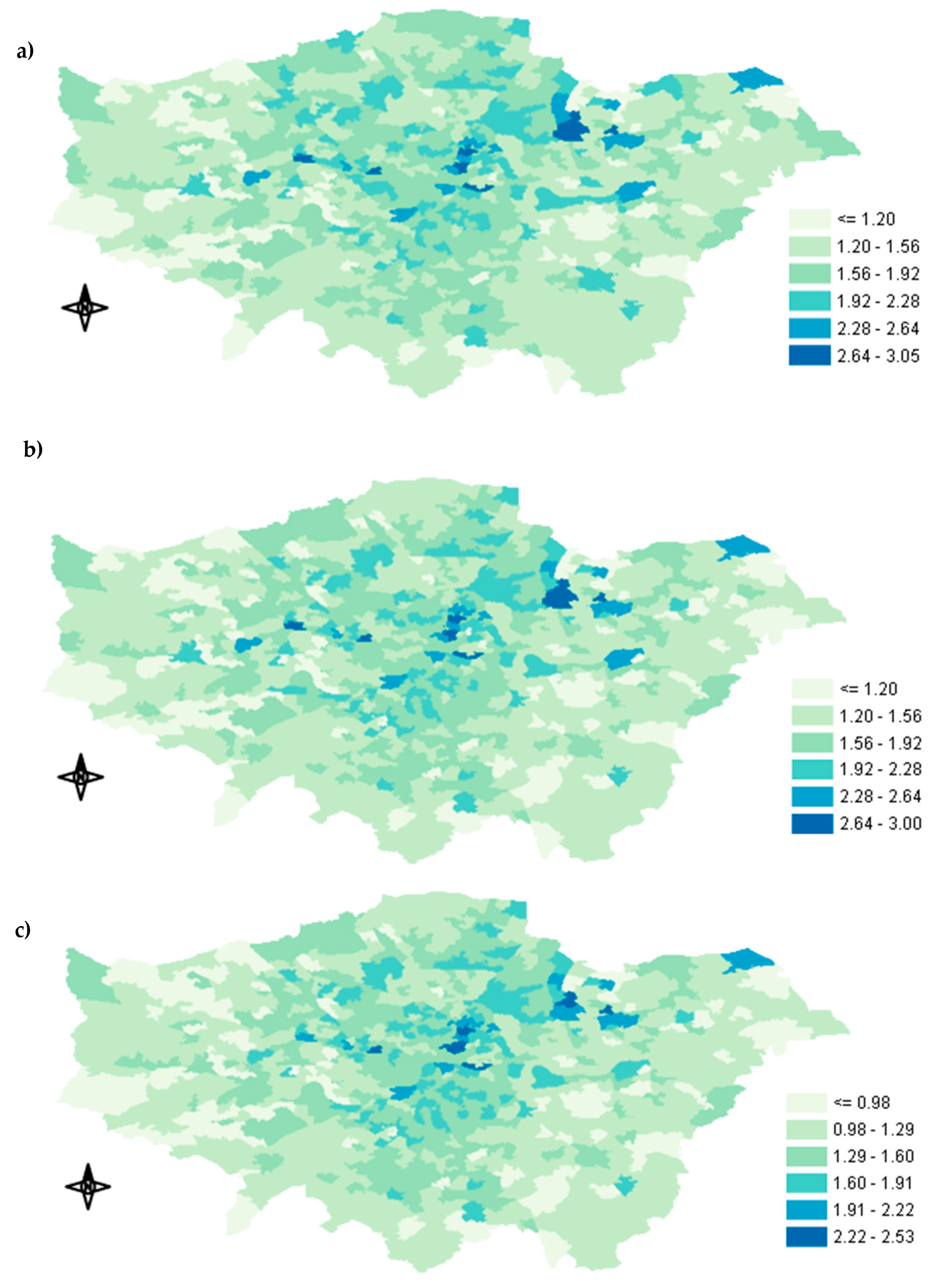
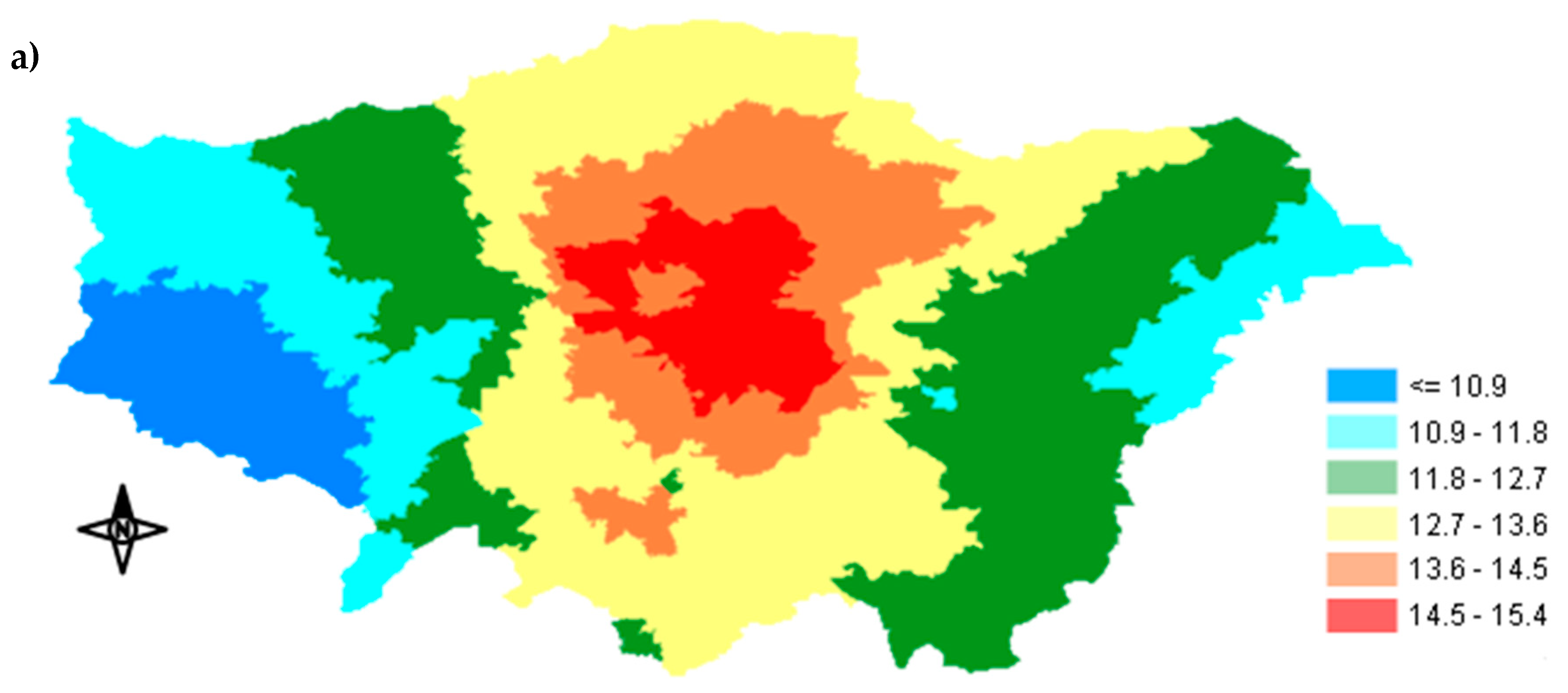
3.1. Exposure Metrics Summary
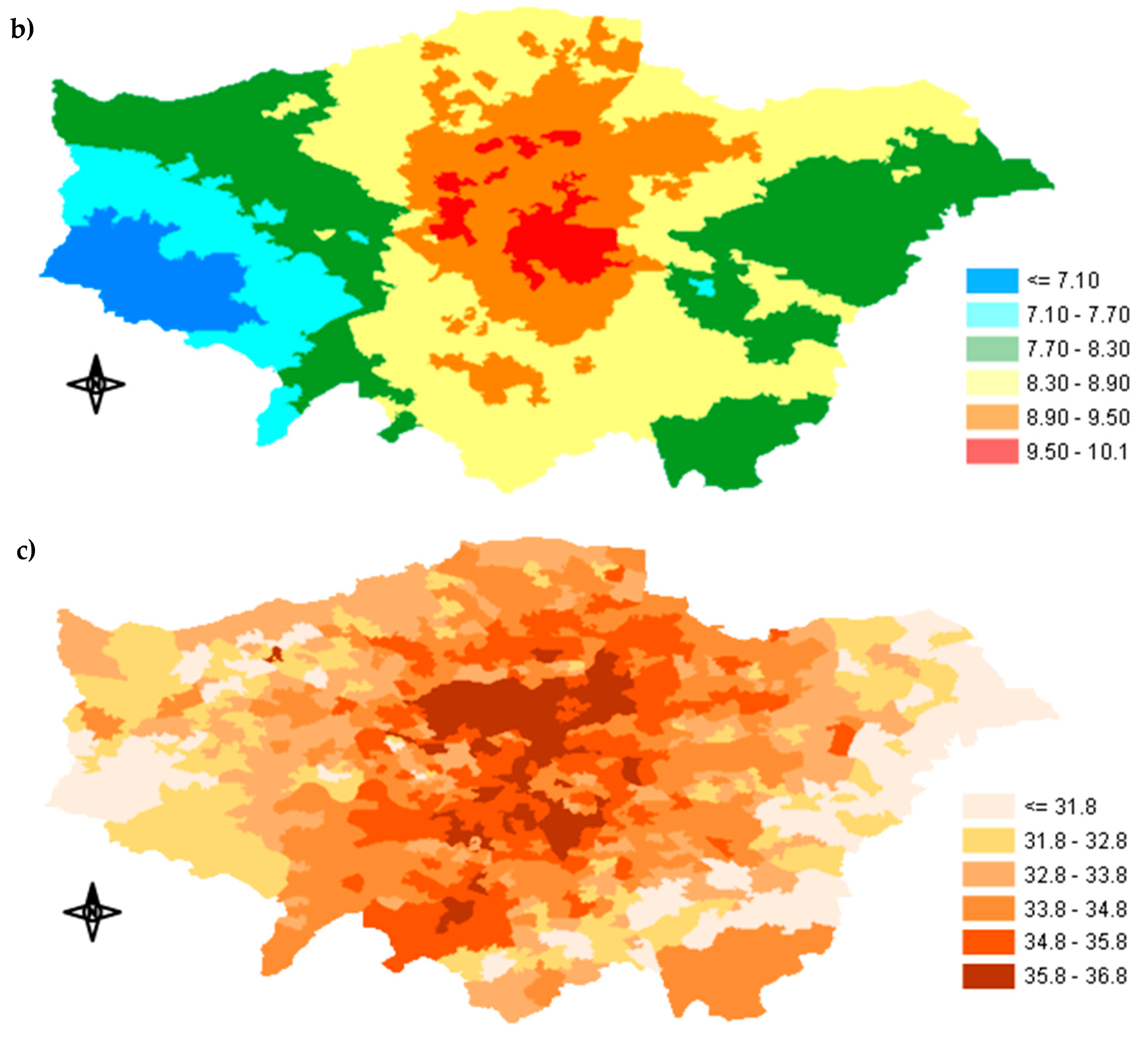
3.2. Epidemiological Implications and Health Impact Misclassification
4. Discussion
Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Spatial Distribution of Exposure Concentration


Appendix A.2. Percentage of Exposure Concentration Reduction across GLA

Appendix A.3. Spatial Distribution of the Predicted Avoided Mortality
References
- Public Health England, COMEAP (2009): Long-term exposure to air pollution: Effect on mortality. GOV.UK Website. Available online: https://www.gov.uk/government/publications/comeap-london-term-exposure-to-air-pollution-effect-on -mortality (accessed on 9 March 2019).
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.; Tchepel, O. Spatial and Temporal Dynamics in Air Pollution Exposure Assessment. Int. J. Environ. Res. Public. Health 2018, 15, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batty, M. Cities as Complex Systems: Scaling, Interactions, Networks, Dynamics and Urban Morphologies. Working Paper. CASA Working Papers (131); Centre for Advanced Spatial Analysis (UCL): London, UK, 2012. [Google Scholar]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in fine particulate air pollution and mortality—Extended follow-up of the Harvard six cities study. Am. J. Respir. Crit. Care 2006, 173, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Dominici, F.; McDermott, A.; Samet, J.M. Mortality in the Medicare Population and Chronic Exposure to Fine Particulate Air Pollution in Urban Centers (2000–2005). Environ. Health Perspect. 2008, 116, 1614–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belleudi, V.; Faustini, A.; Stafoggia, M.; Cattani, G.; Marconi, A.; Perucci, C.A.; Forastiere, F. Impact of Fine and Ultrafine Particles on Emergency Hospital Admissions for Cardiac and Respiratory Diseases. Epidemiology 2010, 21, 414–423. [Google Scholar] [CrossRef]
- Gao, Y.; Chan, E.Y.Y.; Li, L.P.; Lau, P.W.C.; Wong, T.W. Chronic effects of ambient air pollution on respiratory morbidities among Chinese children: A cross-sectional study in Hong Kong. BMC Public Health 2014, 14, 105. [Google Scholar] [CrossRef] [Green Version]
- Collart, P.; Coppieters, Y.; Mercier, G.; Kubuta, V.M.; Leveque, A. Comparison of four case-crossover study designs to analyze the association between air pollution exposure and acute myocardial infarction. Int. J. Environ. Health Res. 2015, 25, 601–613. [Google Scholar] [CrossRef]
- Nikolova, I.; Janssen, S.; Vrancken, K.; Vos, P.; Mishra, V.; Berghmans, P. Size resolved ultrafine particles emission model—A continues size distribution approach. Sci. Total Environ. 2011, 409, 3492–3499. [Google Scholar] [CrossRef]
- Dionisio, K.L.; Isakov, V.; Baxter, L.K.; Sarnat, J.A.; Sarnat, S.E.; Burke, J.; Rosenbaum, A.; Graham, S.E.; Cook, R.; Mulholland, J.; et al. Development and evaluation of alternative approaches for exposure assessment of multiple air pollutants in Atlanta, Georgia. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 581–592. [Google Scholar] [CrossRef]
- Jerrett, M.; Arain, A.; Kanaroglou, P.; Beckerman, B.; Potoglou, D.; Sahsuvaroglu, T.; Morrison, J.; Giovis, C. A review and evaluation of intraurban air pollution exposure models. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Eriksson, C.; Gidhagen, L.; Nilsson, M.E.; Pershagen, G.; Bellander, T. Fine and coarse particulate air pollution in relation to respiratory health in Sweden. Eur. Respir. J. 2013, 42, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.S.; Tsai, M.Y.; Keidel, D.; Gemperli, A.; Ineichen, A.; Hazenkamp-von Arx, M.; Bayer-Oglesby, L.; Rochat, T.; Kunzli, N.; Ackermann-Liebrich, U.; et al. Long-term exposure models for traffic related NO2 across geographically diverse areas over separate years. Atmos. Environ. 2012, 46, 460–471. [Google Scholar]
- Emaus, M.J.; Bakker, M.F.; Beelen, R.M.J.; Veldhuis, W.B.; Peeters, P.H.M.; van Gils, C.H. Degree of urbanization and mammographic density in Dutch breast cancer screening participants: Results from the EPIC-NL cohort. Breast Cancer Res. Treat. 2014, 148, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Burke, J.; Isakov, V.; Lewis, T.; Mukherjee, B.; Robins, T. A Comparison of Exposure Metrics for Traffic-Related Air Pollutants: Application to Epidemiology Studies in Detroit, Michigan. Int. J. Environ. Res. Public Health 2014, 11, 9553–9577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korek, M.J.; Bellander, T.D.; Lind, T.; Bottai, M.; Eneroth, K.M.; Caracciolos, B.; de Faire, U.H.; Fratiglioni, L.; Hilding, A.; Leander, K.; et al. Traffic-related air pollution exposure and incidence of stroke in four cohorts from Stockholm. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Punger, E.M.; West, J.J. The effect of grid resolution on estimates of the burden of ozone and fine particulate matter on premature mortality in the USA. Air Qual. Atmos. Health 2013, 6, 563–573. [Google Scholar] [CrossRef]
- Li, Y.; Henze, D.K.; Jack, D.; Kinney, P.L.J. The influence of air quality model resolution on health impact assessment for fine particulate matter and its components. Air Qual. Atmos. Health 2016, 9, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Fenech, S.; Doherty, R.M.; Heaviside, C.; Vardoulakis, S.; Macintyre, H.L.; O’Connor, F.M. The influence of model spatial resolution on simulated ozone and fine particulate matter for Europe: Implications for health impact assessments. Atmos. Chem. Phys. 2018, 18, 5765–5784. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, A.; Lehtomäki, H.; Rumrich, I.; Karvosenoja, N.; Paunu, V.-V.; Kupiainen, K.; Sofiev, M.; Palamarchuk, Y.; Kukkonen, J.; Kangas, L.; et al. Influence of spatial resolution on population PM2.5 exposure and health impacts. Air Qual. Atmos. Health 2019, 12, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhangar, S.; Mullen, N.A.; Hering, S.V.; Kreisberg, N.M.; Nazaroff, W.W. Ultrafine particle concentrations and exposures in seven residences in northern California. Indoor Air 2011, 21, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Kearney, J.; Wallace, L.; MacNeill, M.; Xu, X.; VanRyswyk, K.; You, H.; Kulka, R.; Wheeler, A.J. Residential indoor and outdoor ultrafine particles in Windsor, Ontario. Atmos. Environ. 2011, 45, 7583–7593. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Spector, D.; Colome, S.; Turpin, B. Determinants of indoor and personal exposure to PM2.5 of indoor and outdoor origin during the RIOPA study. Atmos. Environ. 2009, 43, 5750–5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, L.K.; Burke, J.; Lunden, M.; Turpin, B.J.; Rich, D.Q.; Thevenet-Morrison, K.; Hodas, N.; Ozkaynak, H. Influence of human activity patterns, particle composition, and residential air exchange rates on modeled distributions of PM2.5 exposure compared with central-site monitoring data. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tao, Y.; Kwan, M.-P.; Chai, Y. Assessing Mobility-Based Real-Time Air Pollution Exposure in Space and Time Using Smart Sensors and GPS Trajectories in Beijing. Ann. Am. Assoc. Geogr. 2019, 1–15. [Google Scholar] [CrossRef]
- Ji, W.J.; Zhao, B. Estimating Mortality Derived from Indoor Exposure to Particles of Outdoor Origin. PLoS ONE 2015, 10, e0124238. [Google Scholar] [CrossRef] [Green Version]
- Azimi, P.; Stephens, B. A framework for estimating the US mortality burden of fine particulate matter exposure attributable to indoor and outdoor microenvironments. J. Expo. Sci. Environ. Epidemiol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Fenech, S.; Aquilina, N.J. Trends in ambient ozone, nitrogen dioxide, and particulate matter concentrations over the Maltese Islands and the corresponding health impacts. Sci. Total Environ. 2020, 700, 134527. [Google Scholar] [CrossRef]
- Smith, J.D.; Mitsakou, C.; Kitwiroon, N.; Barratt, B.M.; Walton, H.A.; Taylor, J.G.; Anderson, H.R.; Kelly, F.J.; Beevers, S.D. London Hybrid Exposure Model: Improving Human Exposure Estimates to NO2 and PM2.5 in an Urban Setting. Environ. Sci. Technol. 2016, 50, 11760–11768. [Google Scholar] [CrossRef] [Green Version]
- King’s College London. London Air Quality Network. Available online: https://www.londonair.org.uk/LondonAir/Default.aspx (accessed on 10 December 2018).
- Sacks, J.D.; Lloyd, J.M.; Zhu, Y.; Anderton, J.; Jang, C.J.; Hubbell, B.; Fann, N. The Environmental Benefits Mapping and Analysis Program—Community Edition (BenMAP-CE): A tool to estimate the health and economic benefits of reducing air pollution. Environ. Model. Softw. 2018, 104, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Blangiardo, M.; Gulliver, J. Using Building Heights and Street Configuration to Enhance Intraurban PM10, NOX, and NO2 Land Use Regression Models. Environ. Sci. Technol. 2013, 47, 11643–11650. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sokhi, R.S.; Kitwiroon, N.; Middleton, D.R.; Fisher, B. Performance characteristics of MM5–SMOKE–CMAQ for a summer photochemical episode in southeast England, United Kingdom. Atmos. Environ. 2008, 42, 4870–4883. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.; Shrubsole, C.; Davies, M.; Biddulph, P.; Das, P.; Hamilton, I.; Vardoulakis, S.; Mavrogianni, A.; Jones, B.; Oikonomou, E. The modifying effect of the building envelope on population exposure to PM2.5 from outdoor sources. Indoor Air 2014, 24, 639–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayor of London, London assembley, London Datastore. Available online: https://www.data.london.gov.uk (accessed on 25 January 2019).
- Mayor of London, Transport for London, London Travel Demand Survey (LTDS). Available online: https://www.tfl.gov.uk (accessed on 9 February 2019).
- Song, W.W.; Ashmore, M.R.; Terry, A.C. The influence of passenger activities on exposure to particles inside buses. Atmos. Environ. 2009, 43, 6271–6278. [Google Scholar] [CrossRef]
- Seaton, A.; Cherrie, J.; Dennekamp, M.; Donaldson, K.; Hurley, J.F.; Tran, C.L. The London Underground: Dust and hazards to health. Occup. Environ. Med. 2005, 62, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.D.; Barratt, B.M.; Fuller, G.W.; Kelly, F.J.; Loxham, M.; Nicolosi, E.; Priestman, M.; Tremper, A.H.; Green, D.C. PM2.5 on the London Underground. Environ. Int. 2019, 134, 105–188. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Rodriguez, S.; Plana, F.; Ruiz, C.R.; Cots, N.; Massague, G.; Puig, O. PM 10 and PM2.5 source apportionment in the Barcelona Metropolitan area, Catalonia, Spain. Atmos. Environ. 2001, 35, 6407–6419. [Google Scholar] [CrossRef]
- Johansson, C.; Johansson, P.A. Particulate matter in the underground of Stockholm. Atmos. Environ. 2003, 37, 3–9. [Google Scholar] [CrossRef]
- Altieri, K.E.; Keen, S.L. Public health benefits of reducing exposure to ambient fine particulate matter in South Africa. Sci. Total Environ. 2019, 684, 610–620. [Google Scholar] [CrossRef]
- U.S. EPA Environmental Benefits Mapping and Analysis Program-Community edition: User’s Manual. 2017. Available online: https://www.epa.gov/sites/production/files/2015-04/documents/benmap-ce_user_manual_march_2015.pdf (accessed on 21 January 2019).
- Zhao, D.; Azimi, P.; Stephens, B. Evaluating the Long-Term Health and Economic Impacts of Central Residential Air Filtration for Reducing Premature Mortality Associated with Indoor Fine Particulate Matter (PM2.5) of Outdoor Origin. Int. J. Environ. Res. Public Health 2015, 12, 8448–8479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.; Armstrong, B.; Davies, M.; Ridley, I.; Chalabi, Z.; Shrubsole, C.; Vardoulakis, S.; Wilkinson, P. An Exposure-Mortality Relationship for Residential Indoor PM2.5 Exposure from Outdoor Sources. Climate 2017, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Logue, J.M.; Price, P.N.; Sherman, M.H.; Singer, B.C. A Method to Estimate the Chronic Health Impact of Air Pollutants in US Residences. Environ. Health Perspect. 2012, 120, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA J. Am. Med. Assoc. 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Hinds, W.C.; Kim, S.; Shen, S.; Sioutas, C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos. Environ. 2002, 36, 4323–4335. [Google Scholar] [CrossRef]
- Selmi, W.; Weber, C.; Riviere, E.; Blond, N.; Mehdi, L.; Nowak, D. Air pollution removal by trees in public green spaces in Strasbourg city, France. Urban For. Urban Green. 2016, 17, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Yli-Pelkonen, V.; Scott, A.A.; Viippola, V.; Setala, H. Trees in urban parks and forests reduce O3, but not NO2 concentrations in Baltimore, MD, USA. Atmos. Environ. 2017, 167, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Abhijith, K.V.; Kumar, P.; Gallagher, J.; McNabola, A.; Baldauf, R.; Pilla, F.; Broderick, B.; Di Sabatino, S.; Pulvirenti, B. Air pollution abatement performances of green infrastructure in open road and built-up street canyon environments—A review. Atmos. Environ. 2017, 162, 71–86. [Google Scholar] [CrossRef]
- Strand, M.; Hopke, P.K.; Zhao, W.; Vedal, S.; Gelfand, E.; Rabinovitch, N. A study of health effect estimates using competing methods to model personal exposures to ambient PM2.5. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 549. [Google Scholar] [CrossRef] [Green Version]
- Martins, V.; Moreno, T.; Minguillon, M.C.; Amato, F.; de Miguel, E.; Capdevila, M.; Querol, X. Exposure to airborne particulate matter in the subway system. Sci. Total Environ. 2015, 511, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Tian, L.W.; Thach, T.Q.; Tsui, T.H.; Brauer, M.; Lee, M.; Allen, R.; Yuchi, W.; Lai, P.C.; Wong, P.; et al. Integrating travel behavior with land use regression to estimate dynamic air pollution exposure in Hong Kong. Environ. Int. 2018, 113, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Sokhi, R.S.; Kukkonen, J. An approach to predict population exposure to ambient air PM2.5 concentrations and its dependence on population activity for the megacity London. Environ. Pollut. 2020, 257, 113623. [Google Scholar] [CrossRef] [PubMed]
- Ebelt, S.T.; Wilson, W.E.; Brauer, M. Exposure to Ambient and Nonambient Components of Particulate Matter: A Comparison of Health Effects. Epidemiology 2005, 16, 396–405. [Google Scholar] [CrossRef] [PubMed]



| Tier Models | Exposure Equation | Approach |
|---|---|---|
| Tier model 1 | E = Cout | Outdoors only |
| Tier model 2 | E = Cind | Indoor only |
| Tier model 3 | E = ∑Cout*Fi *xi, | Indoor only (dwellings) |
| Tier model 4 | E = (Cout*tout) +(∑Cout*Fi *xi)*tind + (∑Cout* Fj)* tabg + (Cundg*tundg) | Outdoor + Indoor + Transportation (abg. and undg.) |
| Tier model 5 | E = (Cout* tout) + [(∑Cout*Fi *xi)*tind] + (∑Cout* Fj)* tabg + (Cundg-hvac*tundg-hvac)+(Cdeep-undg *tdeep-undg) | Outdoor + Indoor + Transportation (abg., deep-line + subsurface undg) |
| Dwelling Type | Frequency % | I/O Ratios | Total Average I/O Ratio (All Dwellings) |
|---|---|---|---|
| Bungalow | 1.81 | 0.63 | 0.56 |
| Flat | 50.4 | 0.54 | |
| Terraced | 28.1 | 0.56 | |
| Semi-detached | 14.5 | 0.585 | |
| Detached | 4.06 | 0.585 | |
| Unknown | 1.13 | 0.56 |
| Microenvironments (Groups) | Mode/Place | Time Spent (%) |
|---|---|---|
| Outdoor | Walking | 1.3 |
| Cycling | 0.1 | |
| Transportation (public/private) | Bus | 0.7 |
| Indoor | Car | 1.6 |
| Rail | 0.2 | |
| Underground/DLR | 0.4 | |
| Home, office, other indoor | 95.7 |
| Tier Models | Annual Exposure (μg/m3) | Standard Deviation (+/– μg/m3) |
|---|---|---|
| Tier model 1 | 13.07 | 1.2 |
| Tier model 2 | 7.18 | 0.66 |
| Tier model 3 | 7.26 | 0.66 |
| Tier model 4 | 8.3 | 0.67 |
| Tier model 5 | 8.6 | 0.67 |
| Tier Models | 2.5th percentile | 97.5th percentile | Mean | Decrease (%) |
|---|---|---|---|---|
| Tier models 1–2 | 427 | 2633 | 1541 | |
| Tier models 1–3 | 421 | 2598 | 1521 | 1.95 |
| Tier models 1–4 | 347 | 2151 | 1257 | 18.4 |
| Tier models 1–5 | 324 | 2010 | 1174 | 23.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakos, V.; Luo, Z.; Ewart, I. Quantifying the Health Burden Misclassification from the Use of Different PM2.5 Exposure Tier Models: A Case Study of London. Int. J. Environ. Res. Public Health 2020, 17, 1099. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031099
Kazakos V, Luo Z, Ewart I. Quantifying the Health Burden Misclassification from the Use of Different PM2.5 Exposure Tier Models: A Case Study of London. International Journal of Environmental Research and Public Health. 2020; 17(3):1099. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031099
Chicago/Turabian StyleKazakos, Vasilis, Zhiwen Luo, and Ian Ewart. 2020. "Quantifying the Health Burden Misclassification from the Use of Different PM2.5 Exposure Tier Models: A Case Study of London" International Journal of Environmental Research and Public Health 17, no. 3: 1099. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17031099





