Aflatoxins: Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries
Abstract
:1. Introduction
2. Production, Structural Diversity, and Main Toxicological Properties of Aflatoxins
2.1. Aflatoxin-Producing Molds: Taxonomical Elements
2.2. Physical, Chemical, and Toxicological Properties of Aflatoxins
2.3. Structural Diversity of Aflatoxins
3. Aflatoxin Production and Incidence in Crops and Feeds
3.1. Crop Contamination
3.2. Feed Contamination
4. Toxicity of Aflatoxins and Major Aflatoxicosis Outbreaks
4.1. Aflatoxicoses
4.2. Major Aflatoxicosis Outbreaks
5. Risk Assessment of Aflatoxin Dietary Intake
6. Current Control Means of Crop Contamination with Aflatoxins
6.1. Decontamination
6.2. Prevention
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Benkerroum, N. Retrospective and prospective look at aflatoxin research and development from a practical standpoint. Int. J. Environ. Res. Public Health 2019, 16, 3633. [Google Scholar] [CrossRef] [Green Version]
- Benkerroum, N. Mycotoxins in dairy products: A review. Int. Dairy J. 2016, 62, 63–75. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Safety 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.-L.; Barbier, G.; Coton, E. Filamentous fungi and mycotoxins in cheese: A review. Compr. Rev. Food Sci. Food Safety 2014, 13, 437–456. [Google Scholar] [CrossRef]
- Rohlfs, M. Fungal secondary metabolite dynamics in fungus-grazer interactions: novel insights and unanswered questions. Front. Microbiol. 2014, 5, 788. [Google Scholar] [PubMed] [Green Version]
- Pusztahelyi, T.; Holb, I.J.; Pocsi, I. Secondary metabolites in fungus-plant interactions. Front Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njoroge, S.M.C.; Matumba, L.; Kanenga, K.; Siambi, M.; Waliyar, F.; Maruwo, J.; Machinjiri, N.; Monyo, E.S. Aflatoxin B1 levels in groundnut products from local markets in Zambia. Mycotoxin Res. 2017, 33, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F.; Smedsgaard, J. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography–UV–mass spectrometry methodology. J. Chromatogr. A 2003, 1002, 111–136. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organisation: Lyon (FR), France, 2002; Volume 82, pp. 1–556. [Google Scholar]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Okoth, S.; De Boevre, M.; Vidal, A.; Diana Di Mavungu, J.; Landschoot, S.; Kyallo, M.; Njuguna, J.; Harvey, J.; De Saeger, S. Genetic and toxigenic variability within Aspergillus flavus population isolated from maize in two diverse environments in Kenya. Front. Microbiol. 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.K.; Mack, B.M.; Moore, G.G.; Downey, D.L.; Lebar, M.D.; Joardar, V.; Losada, L.; Yu, J.; Nierman, W.C.; Bhatnagar, D. Whole genome comparison of Aspergillus flavus L-morphotype strain NRRL 3357 (type) and S-morphotype strain AF70. PLoS ONE 2018, 13, e0199169. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Abdin, M.Z.; Ahmad, M.M.; Javed, S. Advances in molecular detection of Aspergillus: An update. Arch. Microbiol. 2010, 192, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesbitt, B.F.; O’Kelly, J.; Sargeant, K.; Sheridan, A.N.N. Aspergillus flavus and turkey X disease: Toxic metabolites of Aspergillus flavus. Nature 1962, 195, 1062–1063. [Google Scholar] [CrossRef] [PubMed]
- Buchanan Jr, R.L.; Ayres, J.C. Effect of sodium acetate on growth and aflatoxin production by Aspergillus parasiticus NRRL 2999. J. Food Sci. 1976, 41, 128–132. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Shotwell, O.L. Intrafungal distribution of aflatoxins among conidia and sclerotia of Aspergillus flavus and Aspergillus parasiticus. Can. J. Microbiol. 1983, 29, 1–5. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Chang, P.K.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004, 70, 6518–6524. [Google Scholar] [CrossRef] [Green Version]
- Mutegi, C.K.; Cotty, P.J.; Bandyopadhyay, R. Prevalence and mitigation of aflatoxins in Kenya (1960-to date). World Mycotoxin J. 2018, 11, 341–357. [Google Scholar] [CrossRef] [Green Version]
- Probst, C.; Callicott, K.A.; Cotty, P.J. Deadly strains of Kenyan Aspergillus are distinct from other aflatoxin producers. Eur. J. Plant Pathol. 2012, 132, 419–429. [Google Scholar] [CrossRef]
- Geiser, D.M.; Dorner, J.W.; Horn, B.W.; Taylor, J.W. The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet. Biol. 2000, 31, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachapulula, P.W.; Akello, J.; Bandyopadhyay, R.; Cotty, P.J. Aflatoxin contamination of groundnut and maize in Zambia: observed and potential concentrations. J. Appl. Microbiol. 2017, 122, 1471–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.-K.; Bennett, J.W.; Cotty, P.J. Association of aflatoxin biosynthesis and sclerotial development in Aspergillus parasiticus. Mycopathologia 2002, 153, 41–48. [Google Scholar] [CrossRef]
- Probst, C.; Schulthess, F.; Cotty, P.J. Impact of Aspergillus section Flavi community structure on the development of lethal levels of aflatoxins in Kenyan maize (Zea mays). J. Appl. Microbiol. 2010, 108, 600–610. [Google Scholar] [CrossRef]
- Townsend, C.A. Aflatoxin and deconstruction of type I, iterative polyketide synthase function. Nat. Prod. Rep. 2014, 31, 1260–1265. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253. [Google Scholar] [CrossRef] [Green Version]
- Theumer, M.G.; Henneb, Y.; Khoury, L.; Snini, S.P.; Tadrist, S.; Canlet, C.; Puel, O.; Oswald, I.P.; Audebert, M. Genotoxicity of aflatoxins and their precursors in human cells. Toxicol. Lett. 2018, 287, 100–107. [Google Scholar] [CrossRef]
- Deshpande, S. Fungal toxins. In Handbook of Food Toxicology; CRC Press: Boca Raton, FL, USA, 2002; pp. 387–456. [Google Scholar]
- Khazaeli, P.; Mehrabani, M.; Heidari, M.R.; Asadikaram, G.; Lari Najafi, M. Prevalence of aflatoxin contamination in herbs and spices in different regions of Iran. Iran J. Public Health 2017, 46, 1540–1545. [Google Scholar]
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsubé, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.B.; Houbraken, J.; Samson, R.A. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Stud. Mycol. 2016, 84, 1–118. [Google Scholar] [CrossRef] [Green Version]
- Rushing, B.R.; Selim, M.I. Effect of dietary acids on the formation of aflatoxin B2a as a means to detoxify aflatoxin B1. Food Addit. Contam. Part A 2016, 33, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Dutton, M.F. Enzymes and aflatoxin biosynthesis. Microbiol. Rev. 1988, 52, 274–295. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.M.; Rushing, B.R.; Selim, M.I. Solvent-dependent transformation of aflatoxin B1 in soil. Mycotoxin Res. 2017, 33, 197–205. [Google Scholar] [CrossRef]
- Swick, R.A. Hepatic metabolism and bioactivation of mycotoxins and plant toxins. J. Anim. Sci. 1984, 58, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.R.; Polan, C.E.; Campbell, T.C. Bovine liver metabolism and tissue distribution of aflatoxin B1. J. Agric. Food Chem. 1977, 25, 1189–1193. [Google Scholar] [CrossRef]
- Yabe, K.; Chihaya, N.; Hatabayashi, H.; Kito, M.; Hoshino, S.; Zeng, H.; Cai, J.; Nakajima, H. Production of M-/GM-group aflatoxins catalyzed by the OrdA enzyme in aflatoxin biosynthesis. Fungal Genet. Biol. 2012, 49, 744–754. [Google Scholar] [CrossRef]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Aflatoxin: Toxicity to dairy cattle and occurrence in milk and milk products—A review. J. Food Prot. 1982, 45, 752–777. [Google Scholar] [CrossRef]
- Trucksess, M.W.; Stoloff, L.; Brumley, W.C.; Wilson, D.M.; Hale, O.M.; Sangster, L.T.; Miller, D.M. Aflatoxicol and aflatoxins B1 and M1 in the tissues of pigs receiving aflatoxin. J. Assoc. Off. Anal. Chem. 1982, 65, 884–887. [Google Scholar]
- Carvajal-Moreno, M.; Vargas-Ortiz, M.; Hernández-Camarillo, E.; Ruiz-Velasco, S.; Rojo-Callejas, F. Presence of unreported carcinogens, aflatoxins and their hydroxylated metabolites, in industrialized Oaxaca cheese from Mexico City. Food Chem. Toxicol. 2019, 124, 128–138. [Google Scholar] [CrossRef]
- Rawal, S.; Coulombe, R.A., Jr. Metabolism of aflatoxin B1 in turkey liver microsomes: The relative roles of cytochromes P450 1A5 and 3A37. Toxicol. Appl. Pharmacol. 2011, 254, 349–354. [Google Scholar] [CrossRef]
- Fan, T.S.; Zhang, G.S.; Chu, F.S. Production and characterization of antibody against aflatoxin Q1. Appl. Environ. Microbiol. 1984, 47, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, M.; Morozumi, S.; Saito, K.; Fujinuma, K.; Nishima, T.; Kasai, N. Interconversion of aflatoxin B1 and aflatoxicol by several fungi. Appl. Environ. Microbiol. 1990, 56, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.J.; Hsieh, D.P. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc. Natl. Acad. Sci. USA 1976, 73, 2241–2244. [Google Scholar] [CrossRef] [Green Version]
- Salhab, A.S.; Edwards, G.S. Production of aflatoxicol from aflatoxin B1 by postmitochondrial liver fractions. J. Toxicol. Environ. Health 1977, 2, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Saito, K.; Kikuchi, Y.; Ibe, A.; Fujinuma, K.; Nishijima, M.; Nishima, T.; Morozumi, S.; Wauke, T.; Hitokoto, H. Aflatoxicol formation by Aspergillus flavus and A. parasiticus. Food Hyg. Safey Sci. 1985, 26, 380–384. [Google Scholar] [CrossRef]
- Detroy, R.W.; Hesseltine, C.W. Transformation of aflatoxin B1 by steroid-hydroxylating fungi. Can. J. Microbiol. 1969, 15, 495–500. [Google Scholar] [CrossRef]
- Frazzoli, C.; Gherardi, P.; Saxena, N.; Belluzzi, G.; Mantovani, A. The hotspot for (Global) one health in primary food production: Aflatoxin M1 in dairy products. Front Public Health 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, M.P.; Applebaum, R.S.; Brackett, R.E.; Marth, E.H. Physical, chemical and biological degradation of mycotoxins in foods and agricultural commodities. J. Food Prot. 1982, 45, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Salhab, A.S.; Hsieh, D.P. Aflatoxicol H1: A major metabolite of aflatoxin B1 produced by human and rhesus monkey livers in vitro. Res. Commun. Chem. Pathol. Pharmacol. 1975, 10, 419–429. [Google Scholar]
- Heathcote, J.G.; Dutton, M.F. New metabolites of Aspergillus flavus. Tetrahedron 1969, 25, 1497–1500. [Google Scholar] [CrossRef]
- Stubblefield, R.D.; Shotwell, O.L.; Shannon, G.M.; Weisleder, D.; Rohwedder, W.K. Parasiticol: A new metabolite from Aspergillus parasiticus. J. Agric. Food Chem. 1970, 18, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kirksey, J.W. Aflatoxin G1 metabolism by Rhizopus species. J. Agric. Food Chem. 1971, 19, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Rodricks, J.V.; Lustig, E.; Campbell, A.D.; Stoloff, L. Aspertoxin, a hydroxy derivative of O-methylsterigmatocystin from aflatoxin-producing cultures of Aspergillus flavus. Tetrahedron Lett. 1968, 2975–2978. [Google Scholar] [CrossRef]
- Pubchem. Explore Chemistry. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 11 February 2020).
- Chemspider. Search and Share Chemistry. 2020. Available online: http://www.chemspider.com (accessed on 11 February 2020).
- Lai, D.Y.; Woo, Y.T.; Arcos, J.C.; Argus, M.F. Difuroxanthone-, Difurocoumarolactone- and Difuroanthraquinone-Type Alkylating Agents: Carcinogenicity and Structure Activity Relationships: Other Biological Properties: Metabolism: Environmental Significance. 1985. Available online: https://www.epa.gov/nscep (accessed on 16 August 2019).
- Dutton, M.F.; Heathcote, J.G. The structure, biochemical properties and origin of the aflatoxins B2a and G2a. Chem. Ind. 1968, 13, 418–421. [Google Scholar]
- Lillehoj, E.B.; Ciegler, A. Biological activity of aflatoxin B2a. Appl. Microbiol. 1969, 17, 516–519. [Google Scholar] [CrossRef] [Green Version]
- IARC (International Agency for Research on Cancer). Chemical agents and related occupations. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon (Fr), France, 2012; Volume 100F, pp. 225–248. [Google Scholar]
- Bbosa, G.; Kitya, D.; Odda, J.; Ogwal-Okeng, J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health (N. Y.) 2013, 5, 14–34. [Google Scholar] [CrossRef] [Green Version]
- Rodricks, J.V.; Henery-Logan, K.R.; Campbell, A.D.; Stoloff, L.; Verrett, M.J. Isolation of a New Toxin from Cultures of Aspergillus flavus. Nature 1968, 217, 668. [Google Scholar] [CrossRef]
- Wood, G.E. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 1992, 70, 3941–3949. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Narrod, C.; Tiongco, M.; Liu, Y. The Health Economics of Aflatoxin: Global Burden of Disease. International Food Policy Research Institute, 2011. Available online: http://www.ifpri.org/publication/health-economics-aflatoxin (accessed on 11 February 2020).
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef]
- Horn, B.W.; Dorner, J.W. Effect of competition and adverse culture conditions on aflatoxin production by Aspergillus flavus through successive generations. Mycologia 2002, 94, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Stasiewicz, M.J.; Hestrin, R.; Morales, L.; Mutiga, S.; Nelson, R.J. Examining environmental drivers of spatial variability in aflatoxin accumulation in Kenyan maize: Potential utility in risk prediction models. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11086–11105. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.; Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. Royal Society Interface 2012, 9, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Sirma, A.J.; Lindahl, J.F.; Makita, K.; Senerwa, D.; Mtimet, N.; Kang’ethe, E.K.; Grace, D. The impacts of aflatoxin standards on health and nutrition in sub-Saharan Africa: The case of Kenya. Glob. Food Secur. 2018, 18, 57–61. [Google Scholar] [CrossRef]
- Anukul, N.; Vangnai, K.; Mahakarnchanakul, W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorolog. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Kaaya, A.N.; Warren, H.L.; Kyamanywa, S.; Kyamuhangire, W. The effect of delayed harvest on moisture content, insect damage, moulds and aflatoxin contamination of maize in Mayuge district of Uganda. J. Sci. Food Agric. 2005, 85, 2595–2599. [Google Scholar] [CrossRef]
- Iqbal, M.; Abbas, M.; Adil, M.; Nazir, A.; Ahmad, I. Aflatoxins biosynthesis, toxicity and intervention strategies: A review. Chem. Int. 2019, 5, 168–191. [Google Scholar]
- Dawd Gashu, D.; Demment, M.W.; Stoecker, B.J. Challenges and opportunities to the African agriculture and food systems. Afr. J. Food Agric. Nutr. Dev. 2019, 19, 14190–14217. [Google Scholar] [CrossRef]
- Njoroge, A.W.; Baoua, I.; Baributsa, D. Postharvest management practices of grains in the Eastern region of Kenya. J. Agric. Sci. 2019, 11. [Google Scholar] [CrossRef]
- Kagot, V.; Okoth, S.; De Boevre, M.; De Saeger, S. Biocontrol of Aspergillus and Fusarium Mycotoxins in Africa: Benefits and Limitations. Toxins (Basel) 2019, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukwago, F.B.; Mukisa, I.M.; Atukwase, A.; Kaaya, A.N.; Tumwebaze, S. Mycotoxins contamination in foods consumed in Uganda: A 12-year review (2006–18). Sci. Afr. 2019, 3, e00054. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, E.S.; Koné, Z.A.; Oyedele, D.A.; Kisito, K.J.; Sika, C.; Koreissi Dembélé, Y.; Eyangoh, S.; et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins (Basel) 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.H.; Tran, S.C.; Le, C.D.; Nguyen, H.-B.T.; Le, P.-T.T.; Le, H.-H.T.; Le, T.D.; Thai-Nguyen, H.-T. Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin A, fumonisin B1, and zearalenone in food from different provinces in Northern Vietnam. Food Control 2020, 112, 107108. [Google Scholar] [CrossRef]
- Hussaini, A.M.; Timothy, A.G.; Olufunmilayo, H.A.; Ezekiel, A.S.; Godwin, H.O. Fungi and some mycotoxins found in mouldy Sorghum in Niger State, Nigeria. World J. Agric. Sci. 2009, 5, 5–17. [Google Scholar]
- Okoth, S.; Kola, M. Market samples as a source of chronic aflatoxin exposure in Kenya. Afr. J. Health Sci. 2012, 20, 56–61. [Google Scholar]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef]
- Ngindu, A.; Kenya, P.; Ocheng, D.; Omondi, T.; Ngare, W.; Gatei, D.; Johnson, B.; Ngira, J.; Nandwa, H.; Jansen, A.; et al. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet 1982, 319, 1346–1348. [Google Scholar] [CrossRef]
- Mahuku, G.; Nzioki, H.S.; Mutegi, C.; Kanampiu, F.; Narrod, C.; Makumbi, D. Pre-harvest management is a critical practice for minimizing aflatoxin contamination of maize. Food Control 2019, 96, 219–226. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A Review on Mycotoxins in Food and Feed: Malaysia Case Study. Compr. Rev. Food Sci. Food Safety 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T. Mycotoxins: Risk assessment and legislation. Toxicol. Lett. 1995, 82–83, 853–859. [Google Scholar] [CrossRef]
- Odera, J.O.; Odera, E.; Githang’a, J.; Walong, E.O.; Li, F.; Xiong, Z.; Chen, X.L. Esophageal cancer in Kenya. Am. J. Dig. Dis. 2017, 4, 23–33. [Google Scholar]
- Sserumaga, J.P.; Ortega-Beltran, A.; Wagacha, J.M.; Mutegi, C.K.; Bandyopadhyay, R. Aflatoxin-producing fungi associated with pre-harvest maize contamination in Uganda. Int. J. Food Microbiol. 2020, 313, 108376. [Google Scholar] [CrossRef] [PubMed]
- Hamidou, F.; Rathore, A.; Waliyar, F.; Vadez, V. Although drought intensity increases aflatoxin contamination, drought tolerance does not lead to less aflatoxin contamination. Field Crop. Res. 2014, 156, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Cardwell, K.F.; Henry, S.H. Risk of exposure to and mitigation of effect of aflatoxin on human health: A West African example. J. Toxicol. Toxin Rev. 2004, 23, 217–247. [Google Scholar] [CrossRef]
- Hell, K.; Cardwell, K.F.; Setamou, M.; Poehling, H.M. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, west Africa. J. Stored Prod. Res. 2000, 36, 365–382. [Google Scholar] [CrossRef]
- Udoh, J.M.; Cardwell, K.F.; Ikotun, T. Storage structures and aflatoxin content of maize in five agroecological zones of Nigeria. J. Stored Prod. Res. 2000, 36, 187–201. [Google Scholar] [CrossRef]
- Ngoko, Z.; Marasas, W.F.O.; Rheeder, J.P.; Shephard, G.S.; Wingfield, M.J.; Cardwell, K.F. Fungal infection and mycotoxin contamination of maize in the Humid forest and the western highlands of Cameroon. Phytoparasitica 2001, 29, 352–360. [Google Scholar] [CrossRef]
- Hell, K.; Cardwell, K.F.; Setamou, M.; Schulthess, F. Influence of insect infestation on aflatoxin contamination of stored maize in four agroecological regions in Benin. Afr. Entomol. 2000, 8, 169–177. [Google Scholar]
- Cardwell, K.F. Mycotoxin Contamination of foods in Africa: Antinutritional factors. Food Nutr. Bull. 2000, 21, 488–492. [Google Scholar] [CrossRef]
- Torres, A.M.; Barros, G.G.; Palacios, S.A.; Chulze, S.N.; Battilani, P. Review on pre- and post-harvest management of peanuts to minimize aflatoxin contamination. Food Res. Int. 2014, 62, 11–19. [Google Scholar] [CrossRef]
- Marete, N.G.; Kanja, W.L.; Mbaria, M.J.; Okumu, O.M.; Ateku, A.P.; Korhonen, H.; Joutsjoki, V. Effects of the Use of good agricultural practices on aflatoxin levels in maize grown in Nandi county, Kenya. Science 2019, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Seetha, A.; Munthali, W.; Msere, H.W.; Swai, E.; Muzanila, Y.; Sichone, E.; Tsusaka, T.W.; Rathore, A.; Okori, P. Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 2017, 33, 323–331. [Google Scholar] [CrossRef] [Green Version]
- World Bank Group. Climate Change Knowledge Portal. 2019. Available online: https://climateknowledgeportal.worldbank.org (accessed on 23 December 2019).
- Climatedata.eu. Climate. 2019. Available online: https://www.climatedata.eu/index.php?lang=en (accessed on 23 December 2019).
- Institute for Veterinary Public Health. World Maps of KöPpen-Geiger Climate Classification. Available online: http://koeppen-geiger.vu-wien.ac.at (accessed on 23 December 2019).
- Niles, E.V.; Norman, J.A.; Pimbley, D. Growth and aflatoxin production of Aspergillus flavus in wheat and barley. Trans. Br. Mycol. Soc. 1985, 84, 259–266. [Google Scholar] [CrossRef]
- Gizachew, D.; Chang, C.-H.; Szonyi, B.; De La Torre, S.; Ting, W.-t.E. Aflatoxin B1 (AFB1) production by Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and temperature. Int. J. Food Microbiol. 2019, 296, 8–13. [Google Scholar] [CrossRef]
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Saïd, S.; Sanchis, V. Effects of temperature, water activity and incubation time on fungal growth and aflatoxin B1 production by toxinogenic Aspergillus flavus isolates on sorghum seeds. Rev. Argent. Microbiol. 2016, 48, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 2019, 293, 472–478. [Google Scholar] [CrossRef]
- Holmquist, G.U.; Walker, H.W.; Stahr, H.M. Influence of Temperature, pH, Water Activity and Antifungal Agents on Growth of Aspergillus flavus and A. parasiticus. J. Food Sci. 1983, 48, 778–782. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Rüfer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010, 26, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Northolt, M.D.; van Egmond, H.P.; Paulsch, W.E. Differences Between Aspergillus flavus Strains in Growth and Aflatoxin B1 Production in Relation to Water Activity and Temperature. J. Food Prot. 1977, 40, 778–781. [Google Scholar] [CrossRef]
- Northolt, M.D.; Verhulsdonk, C.A.H.; Soentoro, P.S.S.; Paulsch, W.E. Effect of Water Activity and Temperature on Aflatoxin Production by Aspergillus parasiticus. J. Milk Food Technol. 1976, 39, 170–174. [Google Scholar] [CrossRef]
- Tennigkeit, T.; Vincent, K. Adaptation of Agricultural Practices to Climate Change in Sub-Saharan Africa (CAADP). Good Agricultural Adaptation Practices: South Africa. Report N°: 11.9757.3–001.00. Freiburg, Germany. 2014. Available online: http://kulima.com/wp-content/uploads/2015/12/CAADP-South-African-Final-Report.pdf (accessed on 26 November 2019).
- Meyer, H.; Skhosana, D.Z.; Motlanthe, M.; Louw, W.; Rohwer, E. Long term monitoring (2014–2018) of multi-mycotoxins in South African commercial maize and wheat with a locally developed and validated LC-MS/MS method. Toxins (Basel) 2019, 11, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Anyamba, A.; Chretien, J.-P.; Britch, S.C.; Soebiyanto, R.P.; Small, J.L.; Jepsen, R.; Forshey, B.M.; Sanchez, J.L.; Smith, R.D.; Harris, R.; et al. Global disease outbreaks associated with the 2015–2016 El Niño event. Sci. Rep. 2019, 9, 1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamachari, K.A.V.R.; Nagarajan, V.; Bhat, R.; Tilak, T.B.G. Hepatitis due to aflatoxicosis: An outbreak in Western India. Lancet 1975, 305, 1061–1063. [Google Scholar] [CrossRef]
- Villers, P. Food safety and aflatoxin control. J. Food Res. 2017, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- PDNA (post-disaster needs assessment). Kenya Post-disaster Needs Assessment 2008–2011 Drought Report: 2012 European Union/United Nations: Government of Kenya. World Bank. 2012. Available online: http://www.gfdrr.org/sites/gfdrr/files/Kenya_PDNA_Final (accessed on 5 June 2019).
- Shank, R.C.; Gordon, J.E.; Wogan, G.N.; Nondasuta, A.; Subhamani, B. Dietary aflatoxins and human liver cancer. III. Field survey of rural Thai families for ingested aflatoxins. Food Cosmet. Toxicol. 1972, 10, 71–84. [Google Scholar] [CrossRef]
- Shank, R.C.; Wogan, G.N.; Gibson, J.B.; Nondasuta, A. Dietary aflatoxins and human liver cancer. II. Aflatoxins in market foods and foodstuffs of Thailand and Hong Kong. Food Cosmet. Toxicol. 1972, 10, 61–69. [Google Scholar] [CrossRef]
- Liverpool-Tasie, L.S.O.; Turna, N.S.; Ademola, O.; Obadina, A.; Wu, F. The occurrence and co-occurrence of aflatoxin and fumonisin along the maize value chain in southwest Nigeria. Food Chem. Toxicol. 2019, 129, 458–465. [Google Scholar] [CrossRef]
- Mutegi, C.; Wagacha, M.; Kimani, J.; Otieno, G.; Wanyama, R.; Hell, K.; Christie, M.E. Incidence of aflatoxin in peanuts (Arachis hypogaea Linnaeus) from markets in Western, Nyanza and Nairobi Provinces of Kenya and related market traits. J. Stored Prod. Res. 2013, 52, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins (Basel) 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitya, D.; Bbosa, G.S.; Mulogo, E. Aflatoxin levels in common foods of South Western Uganda: A risk factor to hepatocellular carcinoma. Eur. J. Cancer Care 2010, 19, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Baluka, S.A.; Schrunk, D.; Imerman, P.; Kateregga, J.N.; Camana, E.; Wang, C.; Rumbeiha, W.K. Mycotoxin and metallic element concentrations in peanut products sold in Ugandan markets. Cogent Food & Agric. 2017, 3, 1313925. [Google Scholar]
- Sirma, A.J.; Senerwa, D.M.; Grace, D.; Makita, K.; Mtimet, N.; Kang’ethe, E.K.; Lindahl, J.F. Aflatoxin B1 occurrence in millet, sorghum and maize from four agro-ecological zones in Kenya. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 10991–11003. [Google Scholar] [CrossRef]
- Menza, N.; Muturi, M.; Kamau, M.L. Incidence, types and levels of aflatoxin in different peanuts varietiesproduced in Busia and Kisii central districts, Kenya. Open J. Med. Microbiol. 2015, 5, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Nyandieka, H.S.; Nyamogoba, H.D.; Nyamwange, C.I. Distribution of aflatoxins and micro organisms in peanut and sunflower seed products and their potential health hazards. Pak. J. Med. Res. 2014, 53, 67–70. [Google Scholar]
- Kang’ethe, E.K.; Sirma, A.J.; Murithi, G.; Mburugu-Mosoti, C.K.; Ouko, E.O.; Korhonen, H.J.; Nduhiu, G.J.; Mungatu, J.K.; Joutsjoki, V.; Lindfors, E.; et al. Occurrence of mycotoxins in food, feed, and milk in two counties from different agro-ecological zones and with historical outbreak of aflatoxins and fumonisins poisonings in Kenya. Food Qual. Saf. 2017, 1, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Gachara, G.W.; Nyamache, A.K.; Harvey, J.; Gnonlonfin, G.J.B.; Wainaina, J. Genetic diversity of Aspergillus flavus and occurrence of aflatoxin contamination in stored maize across three agro-ecological zones in Kenya. Agri. & Food Secur. 2018, 7, 52. [Google Scholar]
- Daniel, J.H.; Lewis, L.W.; Redwood, Y.A.; Kieszak, S.; Breiman, R.F.; Flanders, W.D.; Bell, C.; Mwihia, J.; Ogana, G.; Likimani, S.; et al. Comprehensive assessment of maize aflatoxin levels in Eastern Kenya, 2005–2007. Environ. Health Perspect. 2011, 119, 1794–1799. [Google Scholar] [CrossRef]
- Kiarie, G.; Dominguez-Salas, P.; Kang’Ethe, S.; Grace, D.; Lindahl, J. Aflatoxin exposure among young children in urban low-income areas of Nairobi and association with child growth. Afr. J. Food Agr. Nutr. Dev. 2016, 16, 10967–10990. [Google Scholar] [CrossRef]
- Kang’ethe, E.; Gatwiri, M.; Sirma, A.; Ouko, E.; Mburugu-Musoti, C.; Kitala, P.; Nduhiu, G.; Nderitu, J.; Mungatu, J.; Hietaniemi, V. Exposure of Kenyan population to aflatoxins in foods with special reference to Nandi and Makueni counties. Food Qual. Saf. 2017, 1, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Nyangi, C.; Beed, F.; Mugula, J.; Boni, S.; Koyano, E.; Mahuku, G.; Sulyok, M.; Bekunda, M. Assessment of pre-harvest aflatoxin and fumonisin contamination of maize in Babati District, Tanzania. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11039–11053. [Google Scholar] [CrossRef]
- Kimanya, M.E.; De Meulenaer, B.; Tiisekwa, B.; Ndomondo-Sigonda, M.; Devlieghere, F.; Van Camp, J.; Kolsteren, P. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit. Contam. Part A 2008, 25, 1353–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Mmongoyo, J.A.; Wu, F.; Linz, J.E.; Nair, M.G.; Mugula, J.K.; Tempelman, R.J.; Strasburg, G.M. Aflatoxin levels in sunflower seeds and cakes collected from micro- and small-scale sunflower oil processors in Tanzania. PLoS ONE 2017, 12, e0175801. [Google Scholar] [CrossRef]
- Mngqawa, P.; Shephard, G.S.; Green, I.R.; Ngobeni, S.H.; de Rijk, T.C.; Katerere, D.R. Mycotoxin contamination of home-grown maize in rural northern South Africa (Limpopo and Mpumalanga Provinces). Food Addit. Contam. Part B 2016, 9, 38–45. [Google Scholar] [CrossRef]
- Kamika, I.; Mngqawa, P.; Rheeder, J.P.; Teffo, S.L.; Katerere, D.R. Mycological and aflatoxin contamination of peanuts sold at markets in Kinshasa, Democratic Republic of Congo, and Pretoria, South Africa. Food Addit. Contam. Part B 2014, 7, 120–126. [Google Scholar] [CrossRef]
- Salau, I.A.; Shehu, K.; Muhammad, S.; Umar, R.A. Aflatoxin contamination of stored groundnut kernel in Sokoto State, Nigeria. Greener J. Agric. Sci. 2016, 6, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Oloyede, M.; Williams, A.; Benson, N. Aflatoxin Contamination of Some Edible Grains from Lagos and Ota Markets, Nigeria. Environ. Sci. Technol. 2016, 1, 396–399. [Google Scholar]
- Adebajo, L.O.; Idowu, A.A.; Adesanya, O.O. Mycoflora, and mycotoxins production in Nigerian corn and corn-based snacks. Mycopathologia 1994, 126, 183–192. [Google Scholar] [CrossRef]
- Njumbe Ediage, E.; Hell, K.; De Saeger, S. A comprehensive study to explore differences in mycotoxin patterns from agro-ecological regions through maize, peanut, and cassava products: A case study, Cameroon. J. Agric. Food Chem. 2014, 62, 4789–4797. [Google Scholar] [CrossRef]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Cotty, P.J.; Bandyopadhyay, R. Prevalence of Aflatoxin Contamination in Maize and Groundnut in Ghana: Population Structure, Distribution, and Toxigenicity of the Causal Agents. Plant Dis. 2017, 102, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanvi, D.M.; Lawson-Evi, P.; De Boevre, M.; Goto, C.E.; De Saeger, S.; Eklu-Gadegbeku, K. Natural occurrence of mycotoxins in maize and sorghum in Togo. Mycotoxin Res. 2019, 35, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, M.A.; Oppong, A.; Ofori, K.; Eleblu, J.S.; Ifie, E.B.; Blay, E.; Obeng –Bio, E.; Appiah-Kubi, Z.; Warburton, M.L. Distribution of Aspergillus flavus and aflatoxin accumulation in stored maize grains across three agro-ecologies in Ghana. Food Control 2019, 104, 91–98. [Google Scholar] [CrossRef]
- Van Rensburg, S.J.; Cook-Mozaffari, P.; Van Schalkwyk, D.J.; Van der Watt, J.J.; Vincent, T.J.; Purchase, I.F. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. Br. J. Cancer 1985, 51, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyk, P.S.v.; Merwe, P.J.A.V.d.; Subrahmanyam, P.; Boughton, D. Aflatoxin contamination of groundnuts in Mozambique. Int. Arachis Newsl. 1999, 19, 25–27. [Google Scholar]
- Cambaza, E.; Koseki, S.; Kawamura, S. Aflatoxins in Mozambique: Etiology, Epidemiology and Control. Agriculture 2018, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Reddy, C.S.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31. [Google Scholar] [CrossRef]
- Mohana, D.; Thippeswamy, S.; Abhishek, R.; Shobha, B.; Mamatha, M. Studies on seed-borne mycoflora and aflatoxin B1 contaminations in food based seed samples: Molecular detection of mycotoxigenic Aspergillus flavus and their management. Int. Food Res. J. 2017, 24, 422–427. [Google Scholar]
- Bhat, R.V.; Vasanthi, S.; Rao, B.S.; Rao, R.N.; Rao, V.S.; Nagaraja, K.V.; Bai, R.G.; Prasad, C.A.K.; Vanchinathan, S.; Roy, R.; et al. Aflatoxin b1 contamination in maize samples collected from different geographical regions of India—a multicentre study. Food Addit. Contam. Part B 1997, 14, 151–156. [Google Scholar] [CrossRef]
- Ratnavathi, C.V.; Komala, V.V.; Chavan, U.D. Chapter 3—Mycotoxin Contamination in Sorghum. In Sorghum Biochemistry; Ratnavathi, C.V., Patil, J.V., Chavan, U.D., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 107–180. [Google Scholar]
- Siruguri, V.; Kumar, P.U.; Raghu, P.; Rao, M.V.; Sesikeran, B.; Toteja, G.S.; Gupta, P.; Rao, S.; Satyanarayana, K.; Katoch, V.M.; et al. Aflatoxin contamination in stored rice variety PAU 201 collected from Punjab, India. Indian J. Med. Res. 2012, 136, 89–97. [Google Scholar] [PubMed]
- Koirala, P.; Kumar, S.; Yadav, B.K.; Premarajan, K.C. Occurrence of aflatoxin in some of the food and feed in Nepal. Indian J. Med. Sci. 2005, 59, 331–336. [Google Scholar] [PubMed] [Green Version]
- Quitco, R.T. Aflatoxin studies in the Philippines. In Fungi and mycotoxins in stored products, Proceedings of an international conference held in Bankok, Thailand, 23–26 April 1991; Champ, B.R., Highley, E., Hocking, A.D., Pitt, J.I., Eds.; The Australian Centre for International Africultuaral Research (ACIAR): Bankok, Thailand, 1991; pp. 180–186. [Google Scholar]
- Arim, R.H. Mycotoxin contamination of food and feeds in the Philippines. JSM Mycotoxins 2004, 2003, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Sales, A.C.; Yoshizawa, T. Updated profile of aflatoxin and Aspergillus section Flavi contamination in rice and its byproducts from the Philippines. Food Addit. Contam. Part B 2005, 22, 429–436. [Google Scholar] [CrossRef]
- Panrapee, I.; Phakpoom, K.; Thanapoom, M.; Nampeung, A.; Warapa, M. Exposure to aflatoxin B1 in Thailand by consumption of brown and color rice. Mycotoxin Res. 2016, 32, 19–25. [Google Scholar] [CrossRef]
- Kooprasertying, P.; Maneeboon, T.; Hongprayoon, R.; Mahakarnchanakul, W. Exposure assessment of aflatoxins in Thai peanut consumption. Cogent Food & Agric. 2016, 2. [Google Scholar] [CrossRef]
- Leong, Y.-H.; Ismail, N.; Latif, A.A.; Ahmad, R. Aflatoxin occurrence in nuts and commercial nutty products in Malaysia. Food Control 2010, 21, 334–338. [Google Scholar] [CrossRef]
- Khayoon, W.S.; Saad, B.; Lee, T.P.; Salleh, B. High performance liquid chromatographic determination of aflatoxins in chilli, peanut and rice using silica based monolithic column. Food Chem. 2012, 133, 489–496. [Google Scholar] [CrossRef]
- Semple, R.L.; Frio, A.S.; Hicks, P.A.; Lozare, J.V. Mycotoxin Prevention and Control in Foodgrains. 1991. Available online: http://www.fao.org/3/X5036E/x5036E1b.htm#Mycotoxins%20in%20foodgrains%20in%20some%20Asian%20countries (accessed on 11 February 2020).
- Arzandeh, S.; Selamat, J.; Lioe, H. Aflatoxin in raw peanut kernels Marketed in Malaysia. J. Food Drug Anal. 2010, 18, 44–50. [Google Scholar]
- Soleimany, F.; Jinap, S.; Faridah, A.; Khatib, A. A UPLC–MS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food Control 2012, 25, 647–653. [Google Scholar] [CrossRef]
- Reddy, K.R.; Farhana, N.I.; Salleh, B. Occurrence of Aspergillus spp. and aflatoxin B1 in Malaysian foods used for human consumption. J. Food Sci. 2011, 76, T99–T104. [Google Scholar] [CrossRef] [PubMed]
- Frederick, W.H.; Leinbach, T.R. Southeast Asia 2018: Encyclopædia Britannica, Inc. Available online: https://www.britannica.com/place/Southeast-Asia (accessed on 24 May 2019).
- Reddy, B.N.; Raghvender, C.N. Outbreaks of aflatoxicosis in India. Afr. J. Food Agric. Nutr. Dev. 2007, 7, 1–15. [Google Scholar]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ferrer, E.; Mañes, J. Identification and Quantification of Enniatins and Beauvericin in Animal Feeds and Their Ingredients by LC-QTRAP/MS/MS. Metabolites 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Senerwa, D. Prevalence of aflatoxin in feeds and cow milk from five counties in Kenya. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11004–11021. [Google Scholar] [CrossRef]
- Borutova, R.; Aragon, Y.A.; Nährer, K.; Berthiller, F. Co-occurrence and statistical correlations between mycotoxins in feedstuffs collected in the Asia–Oceania in 2010. Anim. Feed Sci. Tech. 2012, 178, 190–197. [Google Scholar] [CrossRef]
- Anjum, M.A.; Khan, S.H.; Sahota, A.W.; Sardar, R. Assessment of Aflatoxin B1 in commercial poultry feed and feed ingredients. J. Anim. Plant Sci. 2012, 22, 268–272. [Google Scholar]
- Tangendjaja, B.; Rachmawati, S.; Wina, E. Mycotoxin contamination on corn used by feed mills in indonesia. Indones. J. Agric. Sci. 2008, 9, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on health. Indian J. Clin. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef]
- Dilkin, P.; Zorzete, P.; Mallmann, C.A.; Gomes, J.D.; Utiyama, C.E.; Oetting, L.L.; Correa, B. Toxicological effects of chronic low doses of aflatoxin B(1) and fumonisin B(1)-containing Fusarium moniliforme culture material in weaned piglets. Food Chem. Toxicol. 2003, 41, 1345–1353. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Evaluation of certain contaminants in food: eighty-third report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2017; 182 p. [Google Scholar]
- Salhab, A.S.; Edwards, G.S. Comparative in vitro metabolism of aflatoxicol by liver preparations from animals and humans. Cancer Res. 1977, 37, 1016–1021. [Google Scholar]
- Smith, J.W.; Groopman, J.D. Aflatoxins. In Encyclopedia of Cancer (Third Edition); Boffetta, P., Hainaut, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 30–43. [Google Scholar]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Chao, T.C.; Maxwell, S.M.; Wong, S.Y. An outbreak of aflatoxicosis and boric acid poisoning in Malaysia: A clinicopathological study. J. Pathol. 1991, 164, 225–233. [Google Scholar] [CrossRef]
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; De Saeger, S.; Kimanya, M.; Gong, Y.Y.; Simba, A. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Serck-Hanssen, A. Aflatoxin-induced fatal hepatitis? A case report from Uganda. Arch. Environ. Health 1970, 20, 729–731. [Google Scholar] [CrossRef]
- Lye, M.S.; Ghazali, A.A.; Mohan, J.; Alwin, N.; Nair, R.C. An outbreak of acute hepatic encephalopathy due to severe aflatoxicosis in Malaysia. Am. J. Trop. Med. Hyg. 1995, 53, 68–72. [Google Scholar]
- Willis, R.; Mulvihill, J.; Hoofnagle, J. Attempted suicide with purified aflatoxin. Lancet 1980, 315, 1198–1199. [Google Scholar] [CrossRef]
- Mupunga, I.; Mngqawa, P.; Katerere, D.R. Peanuts, aflatoxins and undernutrition in children in sub-saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef] [Green Version]
- Marijani, E.; Nasimolo, J.; Kigadye, E.; Gnonlonfin, G.J.B.; Okoth, S. Sex-related differences in hematological parameters and organosomatic indices of oreochromis niloticus exposed to aflatoxin B diet. Scientifica 2017, 2017, 4268926. [Google Scholar] [CrossRef]
- Monson, S.M.; Coulombe, A.R.; Reed, M.K. Aflatoxicosis: Lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, A.T.; Hirooka, E.Y.; Alvares E Silva, P.L.; Bracarense, A.P.F.R.L.; Flaiban, K.K.M.d.C.; Akagi, C.Y.; Kawamura, O.; Costa, M.C.d.; Itano, E.N. Impact of a single oral acute dose of aflatoxin B₁ on liver function/cytokines and the lymphoproliferative response in C57Bl/6 mice. Toxins (Basel) 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, G.J.; Murcia, H.W. An unusually high production of hepatic aflatoxin B1-dihydrodiol, the possible explanation for the high susceptibility of ducks to aflatoxin B1. Sci. Rep. 2019, 9, 8010. [Google Scholar] [CrossRef] [PubMed]
- Anon. Epidemic of hepatitis in man due to aflatoxicosis. Nutr. Rev. 1976, 34, 45–46. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Outbreak of aflatoxin poisoning--eastern and central provinces, Kenya, January-July 2004. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 790–793. [Google Scholar]
- Outbreak News Today. Aflatoxin Kills 4 Children in Tanzania, Linked to Consumption of Maize. 2017. Available online: http://outbreaknewstoday.com (accessed on 17 June 2019).
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef]
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Appl. Environ. Microbiol. 2007, 73, 2762–2764. [Google Scholar] [CrossRef] [Green Version]
- Infopedia. Nine Emperor Gods Festival. 2019. Available online: https://eresources.nlb.gov.sg/infopedia/articles/SIP_1849_2011–10-21.html (accessed on 23 December 2019).
- Qian, G.; Tang, L.; Lin, S.; Xue, K.S.; Mitchell, N.J.; Su, J.; Gelderblom, W.C.; Riley, R.T.; Phillips, T.D.; Wang, J.-S. Sequential dietary exposure to aflatoxin B1 and fumonisin B1 in F344 rats increases liver preneoplastic changes indicative of a synergistic interaction. Food Chem. Toxicol. 2016, 95, 188–195. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Weekly bulletin on outbreaks and other emergencies: Week 32: 19–25 August 2017. Regional office for Africa, 2017; p. 13. Available online: http://www.who.int/iris/handle/10665/258794 (accessed on 17 June 2019).
- Food and Agriculture Organization of the United Nations; Expert Committee on Food Additives Meeting World Health Organization. International Programme on Chemical, Safety. Safety Evaluation of Certain Food Additives and Contaminants, prepared by the Forty-Ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA)1998; World Health Organization: Geneva, Switzerland. Available online: https://apps.who.int/iris/handle/10665/42092 (accessed on 18 December 2019).
- Shephard, G.S. Risk assessment of aflatoxins in food in Africa. Food Addit. Contam. Part A 2008, 25, 1246–1256. [Google Scholar] [CrossRef]
- Bowers, J.; Brown, B.; Springer, J.; Tollefson, L.; Lorentzen, R.; Henry, S. Risk assessment for aflatoxin: An evaluation based on the multistage model. Risk Anal. 1993, 13, 637–642. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Stacy, S.L.; Kensler, T.W. Global risk assessment of aflatoxins in maize and peanuts: Are regulatory standards adequately protective? Toxicol. Sci. 2013, 135, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO (World Health organization). Global Environment Monitoring System-food Contamination Monitoring and Assessment Programme (GEMS/Food). 2006. Available online: http://www.who.int/foodsafety/chem/gems/en/index1.html (accessed on 20 December 2019).
- Johnson, N.M.; Egner, P.A.; Baxter, V.K.; Sporn, M.B.; Wible, R.S.; Sutter, T.R.; Groopman, J.D.; Kensler, T.W.; Roebuck, B.D. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev. Res. (Phila.) 2014, 7, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.S.; Skipper, P.L.; Peng, X.C.; Groopman, J.D.; Chen, J.S.; Wogan, G.N.; Tannenbaum, S.R. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: Correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis 1988, 9, 1323–1325. [Google Scholar] [CrossRef]
- Wild, C.P.; Hasegawa, R.; Barraud, L.; Chutimataewin, S.; Chapot, B.; Ito, N.; Montesano, R. Aflatoxin-albumin adducts: A basis for comparative carcinogenesis between animals and humans. Cancer Epidemiol. Biomark. Prev. 1996, 5, 179–189. [Google Scholar]
- Gong, Y.Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004, 112, 1334–1338. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Egal, S.; Hounsa, A.; Turner, P.C.; Hall, A.J.; Cardwell, K.F.; Wild, C.P. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: The critical role of weaning. Int. J. Epidemiol. 2003, 32, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin decontamination of food: Cold atmospheric pressure plasma versus “classic” decontamination. Toxins (Basel) 2017, 9, 151. [Google Scholar] [CrossRef]
- Chen, R.; Ma, F.; Li, P.-W.; Zhang, W.; Ding, X.-X.; Zhang, Q.; Li, M.; Wang, Y.-R.; Xu, B.-C. Effect of ozone on aflatoxins detoxification and nutritional quality of peanuts. Food Chem. 2014, 146, 284–288. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Bian, Y.; Chen, Z. Effect of ozone treatment on aflatoxin B1 and safety evaluation of ozonized corn. Food Control 2014, 37, 171–176. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Koliadima, A.; Karaiskakis, G.; Kapolos, J. Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control 2016, 61, 221–226. [Google Scholar] [CrossRef]
- Porto, D.Y.; Trombete, M.F.; Freitas-Silva, O.; de Castro, M.I.; Direito, M.G.; Ascheri, L.J. Gaseous ozonation to reduce aflatoxins levels and microbial contamination in corn grits. Microorganisms 2019, 7, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Jardon-Xicotencatl, S.; Díaz-Torres, R.; Marroquín-Cardona, A.; Villarreal-Barajas, T.; Méndez-Albores, A. Detoxification of aflatoxin-contaminated maize by neutral electrolyzed oxidizing water. Toxins (Basel) 2015, 7, 4294–4314. [Google Scholar] [CrossRef] [Green Version]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application—A review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, L.; Zhu, Y.; Huang, Y.; Hu, X.; Wu, Q.; Nüssler, A.K.; Liu, L.; Yang, W. Current major degradation methods for aflatoxins: A review. Trends Food. Sci. Technol. 2018, 80, 155–166. [Google Scholar] [CrossRef]
- Branà, M.T.; Cimmarusti, M.T.; Haidukowski, M.; Logrieco, A.F.; Altomare, C. Bioremediation of aflatoxin B1-contaminated maize by king oyster mushroom (Pleurotus eryngii). PLoS ONE 2017, 12, e0182574. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, H.; Lee, S.; Lee, J.; Ha, J.; Choi, Y.; Yoon, Y.; Choi, K.-H. Invited review: Microbe-mediated aflatoxin decontamination of dairy products and feeds. J. Dairy Sci. 2017, 100, 871–880. [Google Scholar] [CrossRef]
- Bond, J.Q.; Jungong, C.S.; Chatzidimitriou, A. Microkinetic analysis of ring opening and decarboxylation of γ-valerolactone over silica alumina. J. Catal. 2016, 344, 640–656. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M.; Khan, A.M. Structural analysis and biological toxicity of aflatoxins B1 and B2 degradation products following detoxification by Ocimum basilicum and cassia fistula aqueous extracts. Front. Microbiol. 2016, 7, 1105. [Google Scholar] [CrossRef]
- Lapalikar, G.V.; Taylor, M.C.; Warden, A.C.; Scott, C.; Russell, R.J.; Oakeshott, J.G. F420H2-dependent degradation of aflatoxin and other furanocoumarins is widespread throughout the actinomycetales. PLoS ONE 2012, 7, e30114. [Google Scholar] [CrossRef] [Green Version]
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Biopesticide registration action document Aspergillus flavus AF36. 2003. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006456_3-Jul-03.pdf (accessed on 25 November 2019).
- Dorner, J.W. Development of Biocontrol Technology to Manage Aflatoxin Contamination in Peanuts. Peanut Sci. 2009, 36, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Islam, M.-S.; Callicott, K.A.; Cotty, P.J.; Bandyopadhyay, R. Potential of atoxigenic Aspergillus flavus vegetative compatibility groups associated with maize and groundnut in Ghana as biocontrol agents for aflatoxin management. Front. Microbiol. 2019, 10, 2069. [Google Scholar] [CrossRef]
- Mauro, A.; Garcia-Cela, E.; Pietri, A.; Cotty, J.P.; Battilani, P. Biological control products for aflatoxin prevention in Italy: Commercial field evaluation of atoxigenic Aspergillus flavus active ingredients. Toxins (Basel) 2018, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, R.; Atehnkeng, J.; Ortega-Beltran, A.; Akande, A.; Falade, T.D.O.; Cotty, P.J. “Ground-truthing” efficacy of biological control for aflatoxin mitigation in farmers’ fields in Nigeria: From field trials to commercial usage, a 10-Year study. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Senghor, L.A.; Ortega-Beltran, A.; Atehnkeng, J.; Callicott, K.; Cotty, P.; Bandyopadhyay, R. The atoxigenic biocontrol product Aflasafe SN01 is a valuable tool to mitigate aflatoxin contamination of both maize and groundnut cultivated in Senegal. Plant Disease. 2019. Available online: https://apsjournals.apsnet.org/doi/pdf/10.1094/PDIS-03-19-0575-RE (accessed on 25 November 2019).
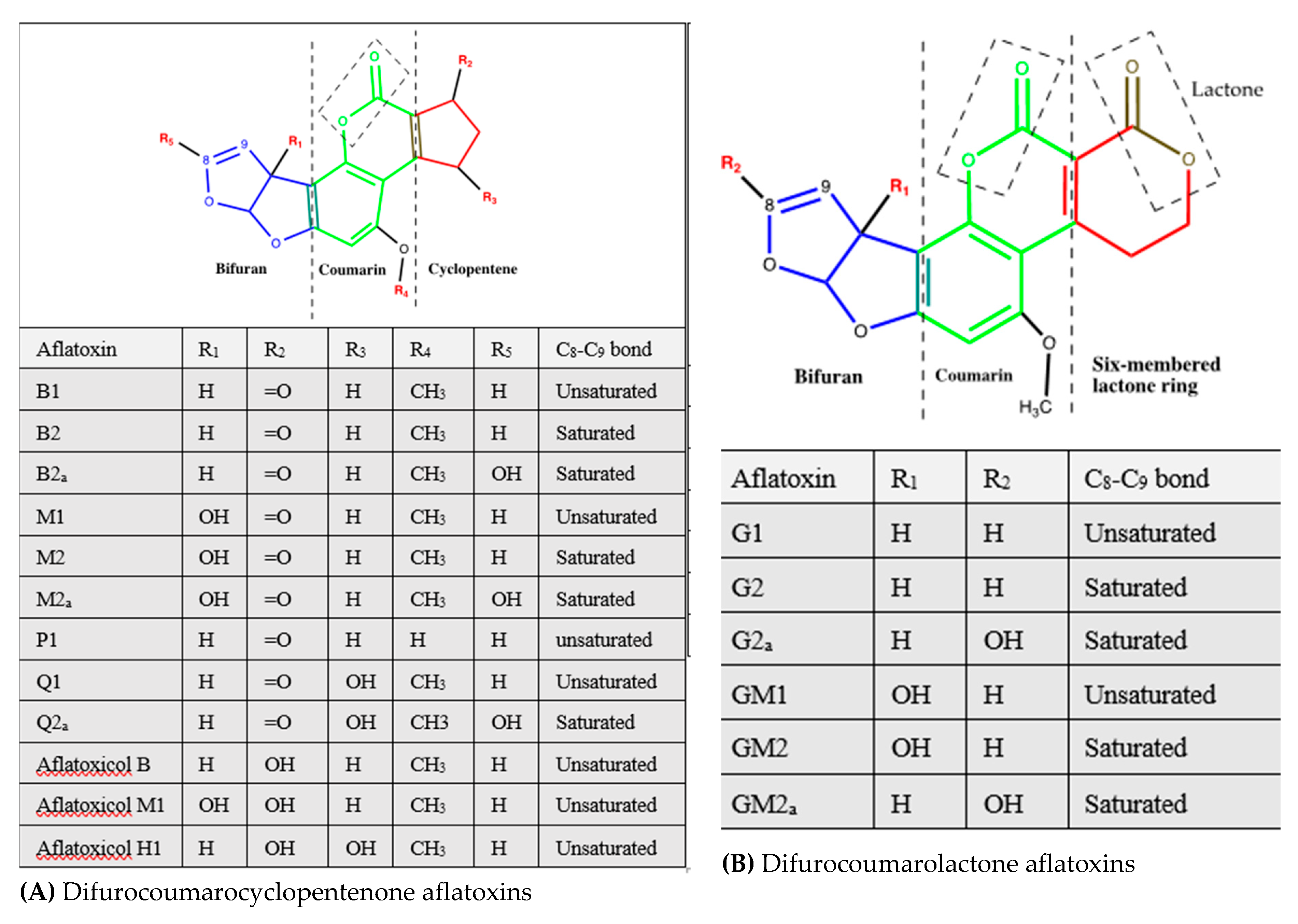
| Aflatoxin | Source | Frequently Contaminated Products | References |
|---|---|---|---|
| Difurocoumarocyclopentenone | |||
| Aflatoxin B1 | Section Flavi: A. flavus, A. pseudotamarii, A. togoensis. A. aflatoxiformans, A. austwickii, A. cerealis, A. arachidicola, A. minisclerotigenes, A. mottae, A. luteovirescens (formerly A. bombycis), A. nomius, A. novoparasiticus, A. parasiticus, A. pipericola, A. pseudocaelatus, A. pseudonomius, and A. sergii, and A. transmontanensis | Cereals (e.g., sorghum, rice, corn, wheat, barely), oil seeds (e.g., cotton seed, oilseed rape, sunflower seed), nuts (e.g., peanuts, groundnut, pistachio), spices (e.g., turmeric, black and red pepper, ginger, allspices), meats, dairy products, fruit juices, dried fruits, eggs, and feeds and foods derived from these products | [11,14,30,31,32] |
| Section Ochraceorosei: A. ochraceoroseus and A. rambellii | |||
| Section Nidulantes: A. astellatus, A. miraensis, A. olivicola, and A. venezuelensis | |||
| Aflatoxin B2 | Section Flavi: A. flavus, A. pseudotamarii, A. aflatoxiformans, A. austwickii, A. cerealis, A. arachidicola, A. minisclerotigenes, A. mottae, A. luteovirescens, A. nomius, A. novoparasiticus, A. parasiticus, A. pipericola, A. pseudocaelatus, A. pseudonomius, A. sergii, A. transmontanensis, Section Ochraceorosei: A. ochraceoroseus and A. rambellii | Cereals (e.g., sorghum, rice, corn, wheat, barely), oil seeds (e.g., cotton seed, oilseed rape, sunflower seed), nuts (e.g., peanuts, groundnut, pistachio), spices (e.g., turmeric, black and red pepper, ginger), meats, dairy products, fruit juices, dried fruits, eggs, and feeds and foods derived from these products | [11,14,30,31,32] |
| Aflatoxin B2a | Hydroxylated metabolite of aflatoxin B1 obtained by water addition to the double bond of the terminal furan under acidic conditions in the liver, the stomach or soil (no evidence for the involvement of specific enzymes) | NA | [33,34,35,36] |
| Naturally produced by A. flavus, and A. parasiticus | |||
| Aflatoxin M1 | Hydroxylated metabolite of aflatoxin B1 by hepatic microsomal mixed-function oxidase system (MFO), mainly cytochromes, in the liver of mammals Produced in vitro from aflatoxin B1 by liver homogenates Naturally produced by A. flavus and A. parasiticus | Milk (including human milk) and dairy products Meat products (kidney, liver) Mouldy groundnut and corn | [30,37,38] |
| Aflatoxin M2 | Hydroxylated metabolite of B2 by hepatic microsomal MFO of mammals Naturally produced by A. parasiticus | Idem as aflatoxin M1 | [30,38] |
| Aflatoxin M2a | Hydration of the terminal furan ring of aflatoxin M1 in dilute acid to yield an hemiketal derivative In vitro in liver homogenates | Milk and dairy products | [39] |
| Aflatoxin P1 | Demethylated metabolite of aflatoxin B1 by liver microsomal oxidase–catalysed O-demethylase | Mainly excreted in the urine (humans and animals). Dairy products | [30,37,40,41] |
| Aflatoxin Q1 | Hydroxylated metabolite of aflatoxin B1 by microsomal enzymes in the liver of higher vertebrates and poultry (main aflatoxin B1 metabolite in monkey) | Assumed to be in edible parts of bovine fed on aflatoxin B1-contaminated feed | [30,37,42] |
| Aflatoxin Q2a | Acid hydration of aflatoxin Q1 | NA | [43] |
| Aflatoxicol (R0) | Metabolite of aflatoxin B1 formed by a reversible reduction of the pentanone group in humans, animals and numerous bacteria and molds In vitro biotransformation of aflatoxin B1 by a soluble cytoplasm reductase system in fish, rat and human liver preparations Naturally produced by A. flavus and A. parasiticus | Mainly avian products (major metabolite in avian species fed on B1-contaminated feed). Dairy products Does not accumulate in edible parts of bovine and swine fed on aflatoxin B1-contaminated feed | [40,41,44,45,46,47,48,49,50] |
| Aflatoxicol M1 | Reduced metabolite of aflatoxin B1, aflatoxin R0, or aflatoxin M1 catalysed by soluble NADPH-dependent reductases in the liver | Milk and dairy products | [30] |
| Aflatoxicol H1 | Reduced metabolite of aflatoxin B1 and aflatoxin Q1 catalysed by soluble NADPH-dependent reductases in the liver | Milk and dairy products | [30,51] |
| Difurocoumarolactone | |||
| Aflatoxin G1 | A. flavusa, A. aflatoxiformans, A. austwickii, A. cerealis, A. arachidicola, A. minisclerotigenes, A. mottae, A. luteovirescens, A. nomius, A. novoparasiticus, A. parasiticus, A. pipericola, A. pseudocaelatus, A. pseudonomius, A. sergii, A. transmontanensis, | Cereals (e.g., sorghum, rice, corn, wheat, barely), oil seeds (e.g., cotton seed, oilseed rape, sunflower seed), nuts (e.g., peanuts, groundnut, pistachio), spices (e.g., turmeric, black and red pepper, ginger), meats, dairy products, fruit juices, dried fruits, eggs, and feeds and foods derived from these products | [11,14,30,31,32] |
| Aflatoxin G2 | A. flavus1, A. aflatoxiformans, A. austwickii, A. cerealis, A. arachidicola, A. minisclerotigenes, A. mottae, A. luteovirescens, A. nomius, A. novoparasiticus, A. parasiticus, A. pipericola, A. pseudocaelatus, A. pseudonomius, A. sergii, and A. transmontanensis | Same as aflatoxin G1 | [11,14,30,31,32] |
| Aflatoxin G2a | Hydroxylated metabolite of aflatoxin G1 obtained by catalytic addition of water to the double bond of the terminal furan under acidic conditions in the liver, the stomach or soil (no evidence for the involvement of specific enzymes). Naturally produced by A. flavus | NA | [30,35,36] |
| Aflatoxin GM1 | Hydroxylated metabolite of aflatoxin G1 by MFO in the liver of mammals Produced in vitro by A. parasiticus fed aspertoxin as a precursor Naturally produced by A. flavus | Milk and dairy products | [38,39,52] |
| Aflatoxin GM2 | Hydroxylated derivative of aflatoxin G2 by MFO in the liver of mammals Produced in vitro by A. parasiticus from dihydro-O-methylsterigmatocystin (DHOMST) Naturally produced by A. flavus and A. parasiticus and yeast | Milk and dairy products | [38,39] |
| Aflatoxin GM2a | Metabolite of aflatoxin GM1in the liver of mammals Hydration of the terminal furan ring of aflatoxin M1 in dilute acid to yield an hemiketal in vitro in liver homogenates | Milk and dairy products | [39] |
| Parasiticol (aflatoxin B3) | A metabolite of aflatoxin G1 from the biodegradation (hydrolysis and decarboxylation reactions) in A. flavus, Rhizopus stolonifer, Rhizopus arrhizus, and Rhizopus oryzae | Idem as aflatoxins B1 and G1 | [14,52,53,54,55] |
| Others | |||
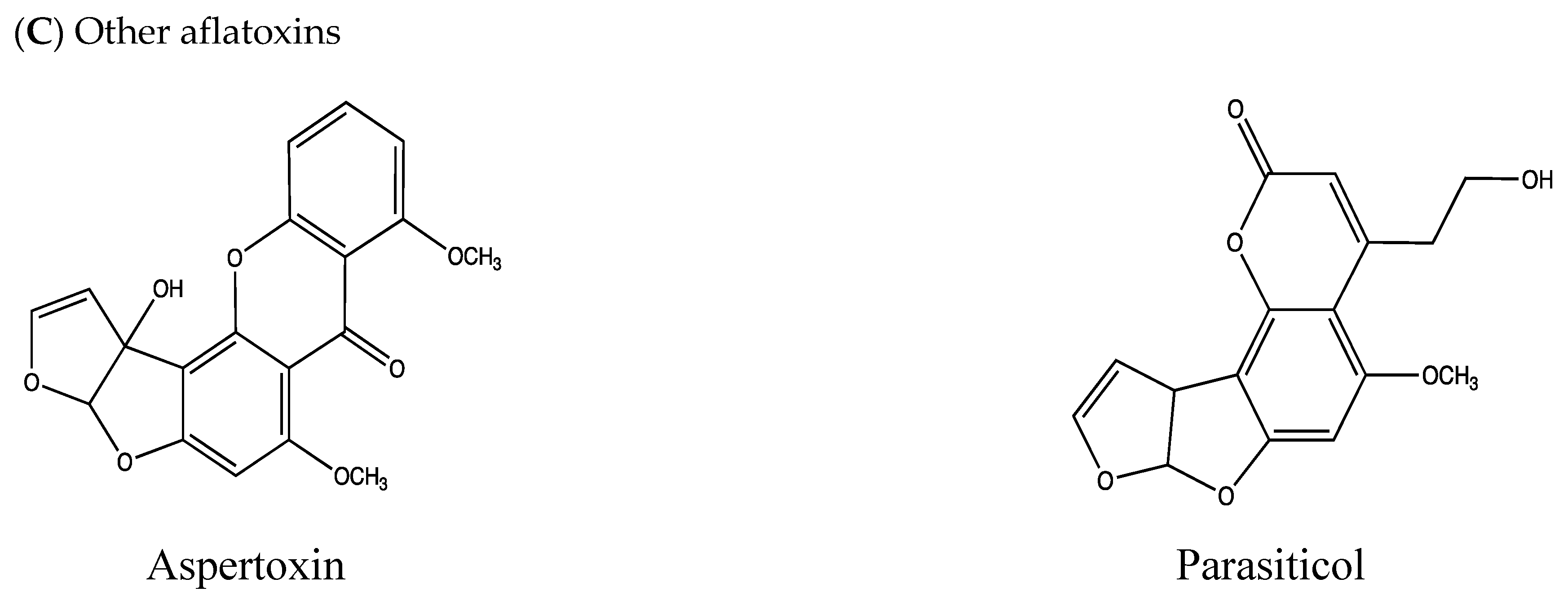
| Parasiticol (aflatoxin B3) | A metabolite of aflatoxin G1 from the biodegradation (hydrolysis and decarboxylation reactions) in A. flavus, Rhizopus stolonifer, Rhizopus arrhizus, and Rhizopus oryzae Naturally produced by A. parasiticus, A. flavus, A. mottae, A. nomius, and A. novoparasiticus | Idem as aflatoxins B1 and G1 | [14,52,53,54,55] |
| Aspertoxin b | A. flavus and A. parasiticus | Mainly vegetal products prone to contamination with A. flavus and A. parasiticus; not considered to be relevant to food products of animal origin | [38,56] |
| Aflatoxin | MW (g/mol) | Formula | Melting Point (°C) a | Toxicity | Adverse Health Effects b | ||
|---|---|---|---|---|---|---|---|
| LD50 (mg/kg bw) | Test Organism | Route | |||||
| Aflatoxin B1 | 312.063 | C17H12O6 | 268.5 | 0.24–60 [59] 3.0 | Various animals and chick embryo Human | Oral, intraperitoneal or injection in chick embryo In vitro experiments | Hepatotoxicity, genotoxicity, carcinogenicity, immuno-toxicity, teratogenicity |
| Aflatoxin B2 | 314.079 | C17H14O6 | 286–289 [59] | 1.7 | Duck | Oral | Week mutagenicity, hepatotoxicity, and carcinogenicity [45] |
| Aflatoxin B2a | 330.074 | C17H14O7 | 240 [59] | >400 μg showed a weak toxicity [60,61] | Ducklings | Oral | Low toxicity (200-fold less than B1) [34,61] |
| Aflatoxin M1 | 328.058 | C17H12O7 | 297–299 | 0.32 1.5 | Duck Rat | Unreported Oral | Hepatotoxicity, nephrotoxicity, carcinogenicity |
| Aflatoxin G2 | 330.074 | C17H14O7 | 237–240 226–229 | 2.5 [39] Weekly mutagenic | Duckling S. typhimurium | Oral Ames’ test | Low toxicity, no evidence for carcinogenicity in animals [30,45,62] |
| Aflatoxin G2a c | 346.069 | C17H14O8 | 243.13 (Predicted) | NA | NA | NA | Low toxicity to inactive (a detoxified form of G1) [30,45] |
| Region Country | Annual Temperature (°C) | Mean Annual Rainfall (mm) | Predominating Climate Types a | ||
|---|---|---|---|---|---|
| Min | Max | Mean | |||
| Sub-Saharan Africa | |||||
| Benin | 25.3 | 30.3 | 27.5 | 1059 | Tropical savanna (Aw) |
| Cameroun | 23.4 | 26.7 | 24.8 | 1614 | Tropical savanna (Aw) |
| Ghana | 25.3 | 29.5 | 27.3 | 1190 | Tropical savanna (Aw) |
| Kenya | 22.6 | 25.9 | 24.3 | 669 | Tropical savanna (Aw) |
| Mali | 21.2 | 33.4 | 28.3 | 333 | Tropical savanna (Aw) |
| Nigeria | 18.5 | 32.4 | 25.4 | 881 | Tropical savanna (Aw) |
| Tanzania | 19.9 | 23.5 | 22.2 | 998 | Tropical savanna (Aw) |
| Togo | 25.0 | 29.5 | 27.0 | 1170 | Tropical savanna (Aw) |
| Uganda | 21.3 | 23.6 | 22.4 | 1200 | Tropical savanna (Aw) |
| Zambia | 17.2 | 25.0 | 22.0 | 976 | Humid subtropical (Cwa) |
| South Africa | 14.6 | 25.9 | 20.3 | 779 | Temperate oceanic (Cfb) |
| Southeast Asia | |||||
| India | 17.0 | 30.0 | 24.1 | 1057 | Tropical savanna (Aw) |
| Indonesia | 22.8 | 30.2 | 28.9 | 2859 | Tropical rainforest (Af) |
| Malaysia | 24.9 | 25.9 | 25.4 | 3059 | Tropical rainforest (Af) |
| Philippines | 24.3 | 27.0 | 25.5 | 2471 | Tropical rainforest (Af) |
| Thailand | 23.0 | 28.9 | 26.3 | 1553 | Tropical savanna (Aw) |
| Vietnam | 20.0 | 27.22 | 26.0 | 149.4 | (Aw) |
| Substrate/ Parameter | Growth | Aflatoxin Production | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. flavus | A. parasiticus | A. flavus | A. parasiticus | ||||||||||
| Max | Min | Opt | Max | Min | Opt | Max | Min | Opt | Max | Min | Opt | ||
| Wheat/ | |||||||||||||
| Temperature | >42.5 | 15 | 35 | - | - | - | 42.5 | 15.0 | 25 | - | - | - | [104] |
| aw | >0.95 | 0.80 | 0.95 | - | - | - | 0.95 | 0.85 | 0.93 | - | - | - | |
| Nyjer seeds a/ | |||||||||||||
| Temperature | NS | NS | 27 | NS | NS | 27 | NS | 20 | 27.0 | NS | 20 | 27.0 | [105] |
| aw | NS | 0.82 | 0.98 | NS | 0.82 | 0.94 | NS | 0.86 | 0.90 | NS | 0.86 | 0.98 | |
| Sorghum/ | |||||||||||||
| Temperature | NS | 15 | 37 | - | - | - | NS | 15 | 37 | - | - | - | [106] |
| aw | NS | <0.91 | 0.97 | - | - | - | NS | 0.94 | 0.97 | - | - | - | |
| Rice/ | |||||||||||||
| Temperature | 42 | 20 | 33 | - | - | - | 37 | <20 | 35 | - | - | - | [107] |
| aw | 0.99 | 0.80 | 0.90 | - | - | - | 0.99 | 0.85 | 0.96 | - | - | - | |
| Sabouraud/ | |||||||||||||
| Temperature | NS | 0.90 | 0.99 | - | - | - | NS | 0.90 | 0.99 | - | - | - | [108] |
| aw | NS | 15 | NS | - | - | - | NS | 15 | NS | - | - | - | |
| Malt Extract-Sucrose/ | |||||||||||||
| Temperature | - | - | - | 42 | 15 | 35 | - | - | - | 40 b 37 c | 17 b 17 c | 37 b 20 c | [109] |
| NS | 12 | 37 | NS | 13 | 32 | 37 | 12 | 31 | NS | 10–13 | 24 | [110,111] | |
| 42 | 15 | 30–35 | - | - | - | - | - | - | 35 | <20 | 25 | [70] | |
| aw | - | - | - | NS | 0.90 | 0.99 | - | - | - | NS NS | 0.90 b 0.90 c | 0.93 b 0.99 c | [109] |
| NS | 0.80 | 0.99 | NS | 0.83 | >0.99 | NS | 0.85 | 0.99 | NS | 0.91 | 0.99 | [110,111] | |
| NS | 0.85 | 0.99 | - | - | - | 0.99 | 0.85 | 0.99 | - | - | - | [70] | |
| Country | Climate Type a | Peanut/Groundnut | Maize | Millet | Sorghum | Sunflower | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Range b (Min–Max) | +ve (%) | Mean Range b (Min–Max) | +ve (%) | Mean Range b (Min–Max) | +ve (%) | Mean Range b (Min–Max) | +ve (%) | Mean Range b (Min–Max) | +ve (%) | |||
| Uganda | Am, Aw, Af | 7.3–221 (2.5–849) | 20–82 | 25.4–75.2 (3.1–510) | 50–100 | 14.0 (NS–NS) | 100 | 11.5–170 (4–472) | 80–100 | - | - | [79,124,125] |
| Kenya | Cbw, Afa, Cfb, Am, Cfb, Aw, As, Cwb, BSh | <LD-1140 (0.1–32,328) c | 7.5–100 | 0.7, 196.3 (0–48,000) | 25–98 | 0.1–66 (1.0–1658) | 21–98 | 0.9–24.5 (<1–265) | 11–100 | 1524 (NS–NS) | NS | [21,84,86,99,122,126,127,128,129,130,131,132,133] |
| Tanzania | Cwb, Aw, Csb, Bsh, Cfa | 0–377.3 (56–3297) | NS | 0.76–106 (1–1081) | 4–28 | - | - | 2.7–93.3 (0–138.7) | NS | 4.9–119 1.4–663 | 50–89 | [100,134,135,136,137] |
| Zambia | Aw, Cwa, BSh, | 4.43–499 (3.9–11,100) | 51–100 | 11–12.0 (3.9–3420) | 42–73 | - | - | - | - | - | - | [7,24] |
| South Africa | Cwb, BSh, Cfb | 6.0–14(74–1416) | 27 d–90 | 1–48 (1–1416) | 6.5–22 d | - | - | - | - | - | - | [113,138,139] |
| Nigeria | BSh, Aw, Am | 6.0–96 (0.9–646) | 26 | 0.6–603 (2.7–1460) | 10–87.5 | 34.3–120.5 (NS–NS) | NS | 165–1245 (0–1164) | 45--64 | [80,82,121,140,141,142] | ||
| Cameroun | Am, Af, Aw | 22–26 (6.0–125) | NS | 47–100 (6–645) | NS | - | - | - | - | - | - | [143] |
| Ghana | Aw, HF, DS SGS, FRT, SVT, RFR | 0.3–145.6 (17–3868) | NS | 6–341 (1–945) | NS 42,100 | - - | - - | 14 (6–19) | - 25 | - - | - - | [144,145,146] |
| Togo | Aw, DS, SGS | 0.3–34.9 (0–168) | NS | 6.8–24.2 (0–157) | - | - | - | - | - | - | - | [145] |
| Benin | Aw | 7.6 (<0.1–105) | 19 | 1.6 (<0.1–20) | 32 | - | - | - | - | - | - | [80] |
| Mali | Aw | 2.2–9.4 (<0.1–246) | 15–29 | - | - | - | - | - | - | - | - | [80] |
| Mozambique | Aw | 5.9–751 (0–2740) | 8.3–92.0 | 2.4–22 (NS-NS) | 67 | - | - | - | - | - | - | [147,148,149] |
| Country | AEZ (Climate Type) a | Peanut/Groundnut | Maize | Rice | Sorghum | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Range b (Min–Max) | +ve (%) | Mean Range (Min–Max) | +ve (%) | Mean Range (Min-Max) | +ve (%) | Mean Range (Min-Max) | +ve (%) | |||
| India | BSh, Aw, Cwa, Am, BWh, Af | 510.7 (NS–NS) | NS | 5–67.3 (0–714) | 21–100 | NS (0.1–308)c | 68–91 | 882 c (0.01–1250) | 82–100 | [150,151,152,153,154] |
| Nepal | Eastern region (Cfa) | NS (54–1806) | 34 | NS (64–859) | 32 | - | - | - | - | [155] |
| The Philippines | Af, Aw | 58 (0–885) | 65 | 39–76 (0.0–1215) | 95 | 1.5 (0–8.7) | 95 | - | - | [156,157,158] |
| Thailand | Aw, Am, Af | 28–1811 (0–12,256) | 43–91 | 196–400 (0–2730) | 39 | 0.8–67 (0–248) | 2–63 | - | - | [72,119,120,159,160,161] |
| Malaysia | NS | 4.3 c–11.3 (1.5–1000) | 16–85 | - | - | 1.75 c (1.1–5.2) | 25–70 | - | - | [162,163,164,165,166] |
| Indonesia | Aw, Af | - | - | 144–464 (NS–490) | 92–100 | - | - | - | - | [163] |
| Vietnam | Aw | 4.96–16.57 (>0.1–362) | 21–26 | 2.62–66.1 (>0.1–1572) | 30–35 | 0.42–2.04 (>0.1–93) | 5.4–12.5 | - | - | [81] |
| Country | Region | Year (Period) | Number of Cases | Number of Deaths (% of Fatality Rate) | Associated Food | Level of Contamination (mg/kg) | Specific Remark | Reference |
|---|---|---|---|---|---|---|---|---|
| India | Western (Rajasthan-Gujarat) | 1974 (October–November) | 397 | 106 (26.7) | Maize | 6.3–15.6 | Heavy unseasonal rain after drought and faulty storage conditions | [116] |
| Kenya | East-Central(Makueni) | 1981 (March–June) | 20 | 12 (60) | Maize | 3.2 and 12.0 | Rain shortage in the year preceding the outbreak followed by prolonged high rainy season and faulty storage conditions | [85] |
| East-Central (Makueni-Kitui-Machakos-Thika) | 2004 (January–July) a | 317 | 125 (39.4) | Maize | 1.0–46.4 | First study relating aflatoxin-albumin adduct to human aflatoxicosis and its use as a biomarker | [84,193] | |
| Malaysia | Perak state | 1988 | 17 b | 13 c (76.5) | Chinese noodles d | NS | Possible additive effect of boric acid and aflatoxin | [182,185] |
| Tanzania | Central | 2016 (May–November) | 67 | 20 (30) | Maize | 10–51,100 | Possible additive effect of fumonisins High titres of aflatoxin-albumin adduct in the serum of patients used as evidence for the aflatoxicosis | [183] |
| Northeast | 2017 (June–July) | 8 | 4 (50) | Maize | NA | Evidence based on symptoms and consumption of maize reported to have been inadequately stored | [194] |
| Country | Product | Exposure a | R b | MOE c |
|---|---|---|---|---|
| Kenya | Maize (commercial) | 133 | 11 | 1.3 |
| Maize (rural market) | 353 | 29.2 | 0.5 | |
| Ghana | Kenkey d | 850 | 70.1 | 0.2 |
| Botswana | Peanut butter | 23 | 1.9 | 7.4 |
| Benin | Yam chips e | 105 | 8.7 | 1.6 |
| The Gambia | Maize | 3.6 | 0.3 | 47.2 |
| Millet | 30 | 2.4 | 5.7 | |
| Sorghum | 1.4 | 0.1 | 121.4 | |
| Rice | 14 | 1.1 | 12.1 | |
| Groundnut | 16 | 1.3 | 10.6 |
| Cluster Diet | Country | MTL a (ng/g) | MAL b (ng/g) | |
|---|---|---|---|---|
| Risk 1/100,000 | Risk 1/10,000 | |||
| G | China | 40 | 7 | 66 |
| India | 30 | 13 | 129 | |
| Indonesia | 20 | 13 | 127 | |
| Malaysia | 15 c | 9 | 89 | |
| Nepal | 40 | 17 | 169 | |
| Sri Lanka | NA | 14 | 138 | |
| Thailand | NA | 9 | 94 | |
| Vietnam | NA | 7 | 66 | |
| I | Kenya | 20 | 1 | 9 |
| Malawi | 10 | 1 | 12 | |
| Mozambique | 10 b | 2 | 17 | |
| South Africa | 10 | 2 | 20 | |
| Tanzania | 10 | 2 | 16 | |
| Zimbabwe | 10 | 1 | 10 | |
| J | Nigeria | NA | 2 | 24 |
| Sudan | NA | 4 | 42 | |
| L | The Phillippines | 20 | 7 | 68 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkerroum, N. Aflatoxins: Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17041215
Benkerroum N. Aflatoxins: Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries. International Journal of Environmental Research and Public Health. 2020; 17(4):1215. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17041215
Chicago/Turabian StyleBenkerroum, Noreddine. 2020. "Aflatoxins: Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries" International Journal of Environmental Research and Public Health 17, no. 4: 1215. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17041215





